Germylenes are heavier carbene analogues. They have been used as ligands for late-transition-metal complexes that can serve as catalysts for hydroformylation, cyclotrimerization, and C–H borylation reactions. It has been proposed that their catalytic activity may be higher than that of the phosphine analogues as a result of the stronger σ-donor properties of the Ge atom. Studies dealing with the tuning of the electronic properties of germylene complexes could, therefore, be useful.
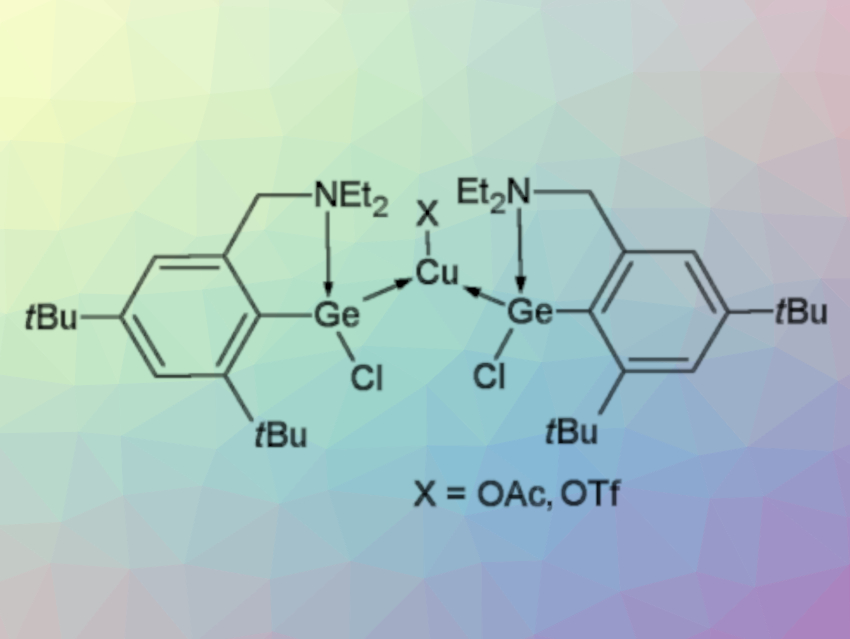
Roman Jambor, University of Pardubice, Czech Republic, and colleagues have developed a synthesis of monomeric Cu complexes coordinated by two Ge atoms that can undergo selective substitution reactions to introduce different groups at the Cu or Ge atoms. The monomeric Cu complexes used are stabilized by the N→Ge coordinated germylene L(Cl)Ge (L = 2-Et2NCH2-4,6-tBu2-C6H2, example complex pictured above).
The team first prepared the complex [L(Cl)Ge]2CuI via a reaction of the corresponding germylene with CuI (pictured below). The resulting monomeric copper complex can be reacted with AgOAc or AgOTf to replace the iodide ligand at copper and give [L(Cl)Ge]2CuX (X = OAc,OTf). In contrast, when [L(Cl)Ge]2CuI is reacted with K[BEt3H], the Cl atoms in the ligand are replaced and the complex [L(H)Ge]2CuI is obtained.
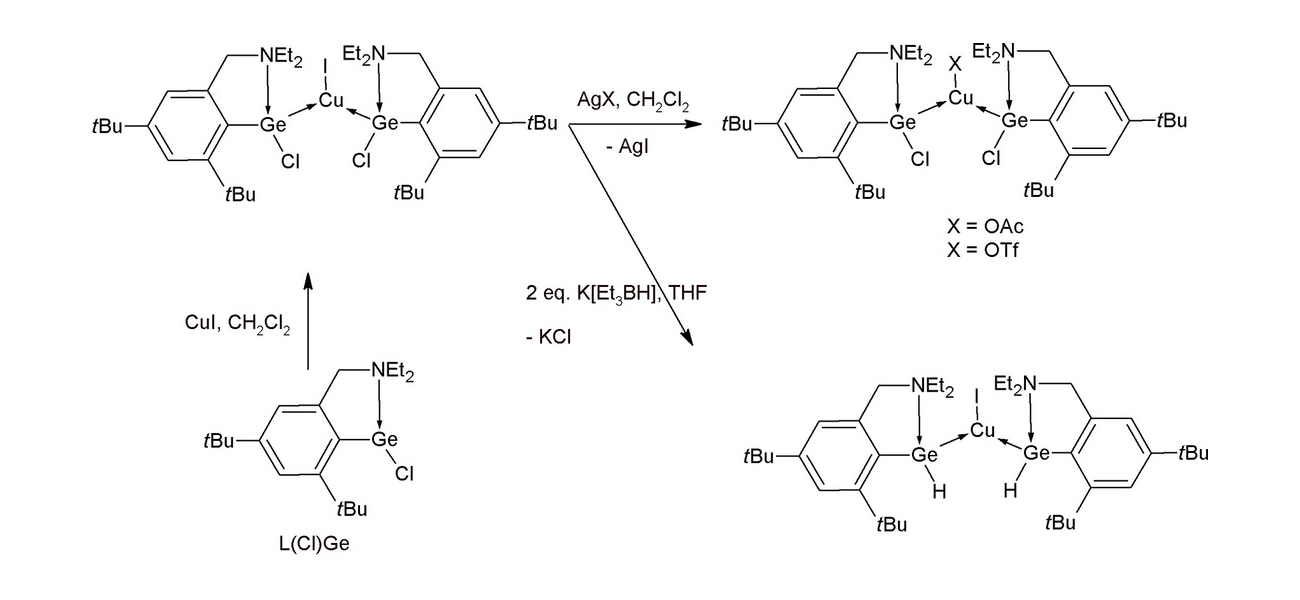
[L(H)Ge]2CuI is a rare structurally characterized monomeric transition-metal complex supported by two neutral organogermylene hydrides. Overall, the work demonstrates that it is possible to tune the electronic properties of the Ge or Cu atoms in copper–germylene complexes by introducing different substituents. This could be useful for the development of catalysts.
- N→Ge coordinated germylenes as ligands for monomeric Cu complexes,
Dominik Vítek, Libor Dostál, Aleš Růžička, Tomáš Mikysek, Roman Jambor,
Eur. J. Inorg. Chem. 2021.
https://doi.org/10.1002/ejic.202100523