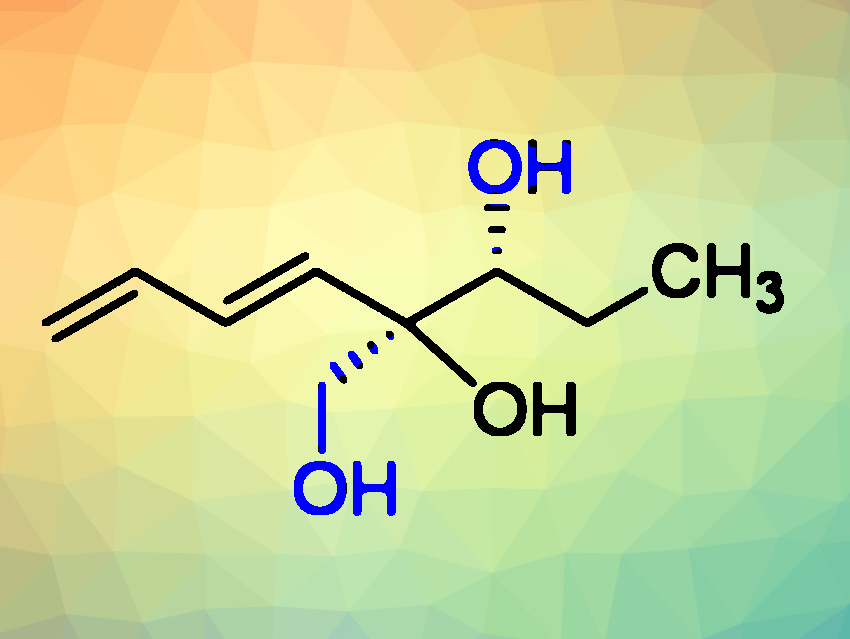
Shivajirao L. Gholap, Indian Institute of Technology Delhi, New Delhi, and colleagues have performed the first asymmetric total synthesis of (+)-isostreptenol III (pictured). (+)-Isostreptenol III is a metabolite isolated from the organic extract of the terrestrial fungus Penicillium purpurogenum MM. This bioactive compound contains a diene unit and two contiguous stereocenters, of which one is a quaternary stereocenter. Until now, there had been no total synthesis of (+)-isostreptenol III to confirm its structure.
The team started from commercially available D-ribose, which was converted to a lactol, followed by the stereoselective installation of hydroxymethyl group and the introduction of an aldehyde group. Further key steps were a diastereoselective Wittig olefination and the introduction of an epoxide group (pictured below, MOM = methoxymethyl). Finally, the team synthesized (+)-isostreptenol III using an epoxide opening with the Grignard reagent methyl magnesium bromide in the presence of copper(I) iodide.
.jpg)
Based on spectroscopic data, the team revised the absolute stereochemistry of the isolated natural product from the proposed (3R,4S)-(+)-isostreptenol III to 3R,4R-(+)-isostreptenol III. This was confirmed by synthesis of its enantiomer 3S,4S-(–)-isostreptenol III, which has an optical rotation that is in excellent agreement with that of the isolated natural (+)-isostreptenol, but with the opposite sign.
- First total synthesis and structure revision of (+)-Isostreptenol III,
Kapil Sharma, Naresh Surineni, Anu Dalal, Shivajirao L Gholap,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100655


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
