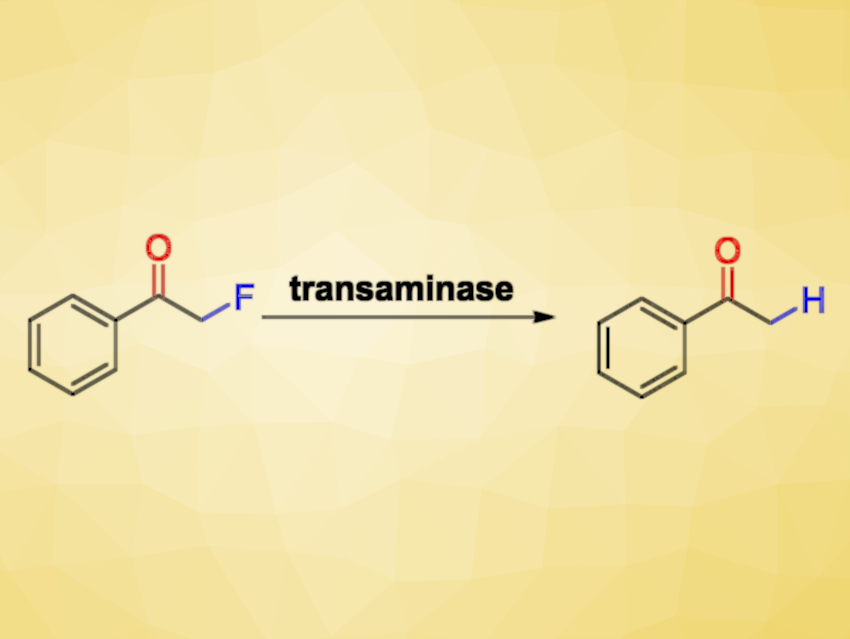
Transaminases (TAs) are enzymes that can be useful, e.g., as catalysts for the synthesis of chiral amines starting from carbonyl compounds. Fluorinated organic compounds are interesting due to the useful properties that the fluorine atoms can confer. However, because of their inert nature, the degradation of such halogenated compounds can be difficult. While there are many transition-metal-based approaches to reductively defluorinate aromatic fluoroarenes, the cleavage of C−F bonds in aliphatic compounds is not so well-developed.
Iván Lavandera, University of Oviedo, Spain, Wolfgang Kroutil, University of Graz, Austria, Gideon Grogan, University of York, UK, and colleagues have found that TAs show an unexpected reactivity and can catalyze the substitution of a fluorine by a hydrogen atom, or hydrodefluorination, of monofluorinated ketones (pictured above). The selective hydrodefluorination of α-fluoroketones or aldehydes is a difficult transformation since these carbonyl compounds are highly reactive and both the C−F bond and the C=O bond can potentially be reduced.
The reaction uses Chromobacterium violaceum and Arthrobacter sp. TAs and produces the desired defluorinated derivatives in an aqueous medium under very mild conditions. It requires a stoichiometric amount of an amine, e.g., 2-propylamine, as a reagent and gives, e.g., acetone as well as ammonia and hydrogen fluoride as byproducts. The transformation can be performed in a chemo-, regio- and stereoselective manner.
- The Reactivity of α‐Fluoroketones with PLP Dependent Enzymes: Transaminases as Hydrodefluorinases,
Marina García‐Ramos, Aníbal Cuetos, Wolfgang Kroutil, Gideon Grogan, Iván Lavandera,
ChemCatChem 2021.
https://doi.org/10.1002/cctc.202100901