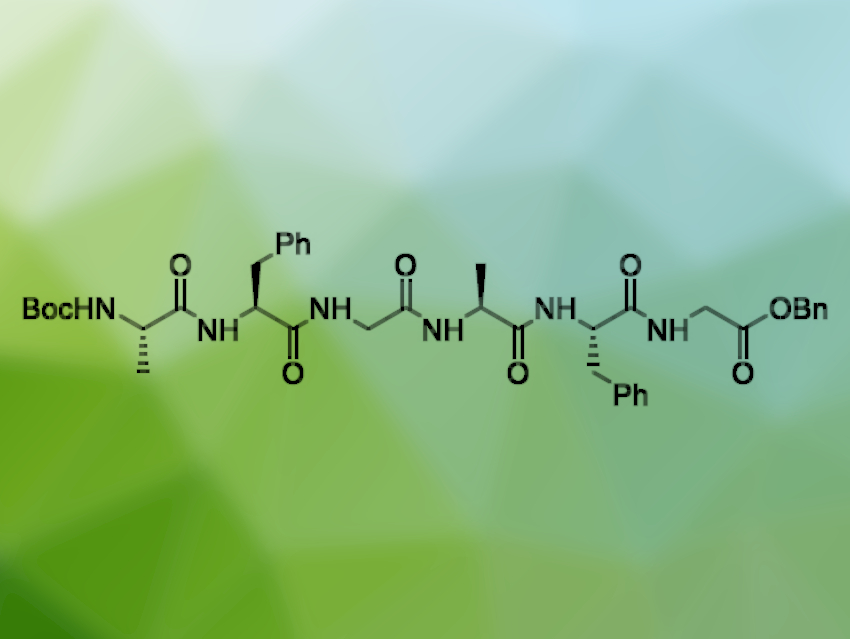
The chemical synthesis of longer peptide chains can be challenging and often requires large amounts of harmful solvents such as dimethylformamide (DMF). Solventless methods could help to alleviate this problem and reduce waste. The use of ball mills, a technique widely employed in mechanochemistry, enables a very efficient mixing of reactants that can allow a reduction in the use of harmful chemicals.
Thomas-Xavier Métro, University of Montpellier, France, and colleagues have synthesized the longest peptide chain with a precisely controlled amino acid sequence made in a ball mill to date: the hexapeptide Boc-(Ala-Phe-Gly)2-OBn (pictured), produced in an amount of 1.7 g in five linear steps with an overall yield of 77 % (Boc = tert-butyloxycarbonyl, Bn = benzyl). The team used a fragment coupling approach via an Ala-Phe-Gly monomer.
They started from Boc-protected phenylalanine, which was reacted with a benzyl-protected glycine in a vibratory ball mill in the presence of ethyl cyanohydroxyiminoacetate (oxyma), NaH2PO4, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDCI), and EtOAc as a liquid additive to give the dipeptide Boc-Phe-Gly-OBn. The amine was deprotected and the dipeptide was coupled with Boc-protected alanine under the same conditions to give Boc-Ala-Phe-Gly-OBn. Finally, another deprotection step at the amino group allowed a coupling with Boc-Ala-Phe-Gly-OH in the ball mill to give the desired hexapeptide.
- Gram‐Scale Synthesis of a Hexapeptide by Fragment Coupling in a Ball Mill,
Yves Yeboue, Nadia Rguioueg, Gilles Subra, Jean Martinez, Frédéric Lamaty, Thomas-Xavier Métro,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100839