Carrying out reactions in superacidic systems, e.g. HF/MF5 (M = As, Sb), offers the possibility to prepare cationic species that are not stable under common conditions. Usually, the prepared species are destabilized compared to the corresponding neutral compound due to the protonation. They are obtained as salts that are sensitive towards air and temperature. Until now, little was known about the reaction behavior of cyclic esters in superacidic media.
Andreas J. Kornath, Ludwig Maximilian University, Munich, Germany, and colleagues have investigated ethylene carbonate, C3H4O3 (a simple representative of cyclic esters), in the above-mentioned superacidic systems. The team synthesized protonated species of ethylene carbonate, [C3H5O3][AsF6], [C3H5O3][SbF6], and [C3H5O3][Sb2F11]. For this purpose, ethylene carbonate was reacted in the superacids HF/MF5 at low temperatures (−40 °C), with an excess of anhydrous HF serving a dual purpose as reactant and solvent.
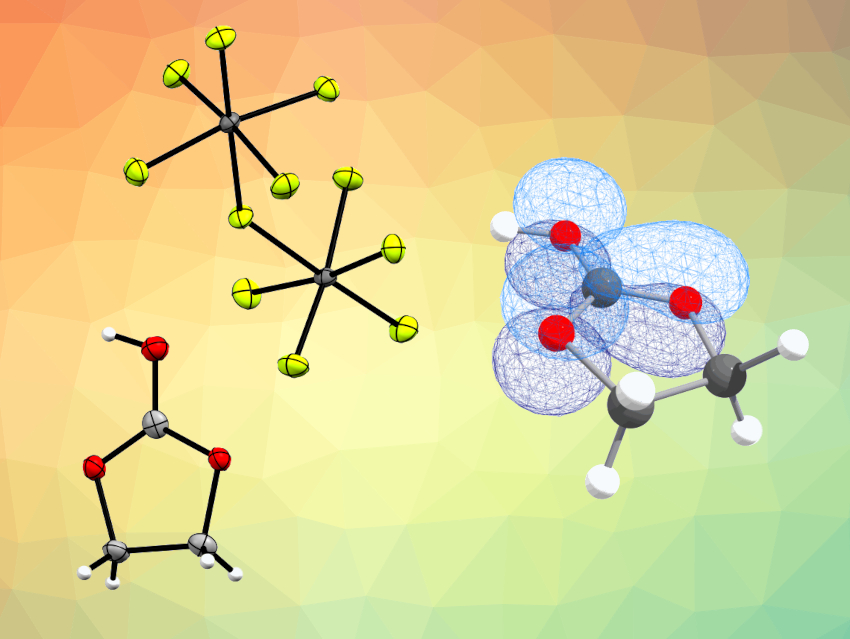
The compounds were characterized using IR and Raman spectroscopy and, in the case of [C3H5O3][Sb2F11], using X-ray structure analysis. The crystal structure of the antimony species [C3H5O3][Sb2F11] (pictured) shows a planar CO3 moiety with almost equal CO bond lengths. Natural bond orbital (NBO) calculations confirmed the formation of a remarkable delocalized 6π-electron system, i.e., protonated ethylene carbonate is a cation where the positive charge is strongly delocalized over the CO3 moiety.
- Protonated Ethylene Carbonate: A Highly Resonance‐Stabilized Cation,
Stefanie Beck, Christoph Jessen, Andreas J. Kornath,
ChemistryOpen 2021.
https://doi.org/10.1002/open.202100229