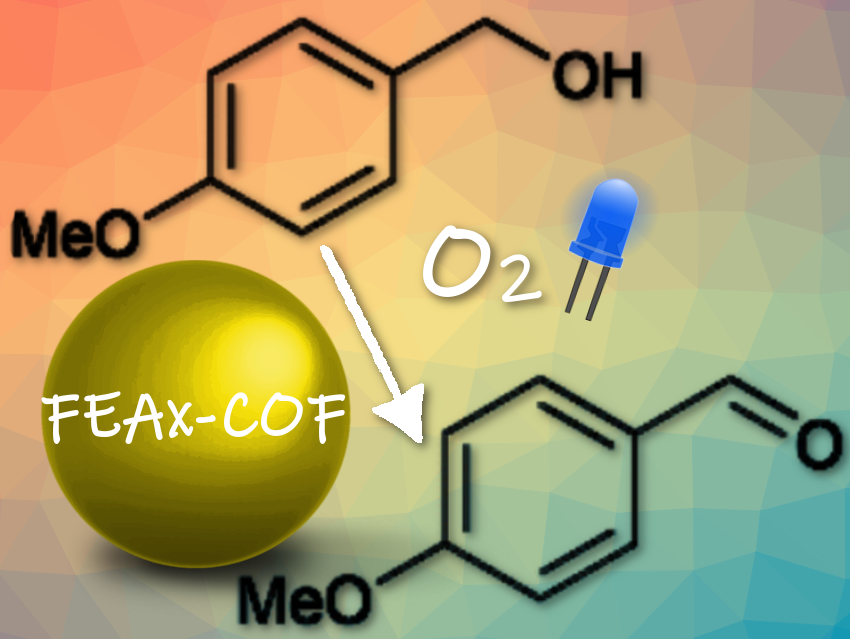
Molecular oxygen is the cheapest and most abundant oxidizing agent, and it is used by organic chemists to perform sustainable and economical oxidation reactions. These reactions are often powered by light. However, the best photocatalysts are metal-based and costly, and fully organic dyes only function in homogeneous reactions, fueling the need for versatile, metal-free, and heterogeneous alternatives.
As such an alternative, Bettina V. Lotsch, Max Planck Institute for Solid State Research, Stuttgart, Germany, and colleagues have developed a solid-state, fully organic photocatalyst. It consists of a porous covalent organic framework (COF) based on isomers of flavin, a natural dye, and redox-active molecule.
Flavins: Ubiquitous, Yellow-Colored Hydrogen Transfer Units
The flavin molecular unit is the redox-active part of vitamin B2, riboflavin. Flavins absorb blue light, and they perform single-electron transfer processes. Replacing the ribityl unit in flavin with hydrogen creates isoalloxazines, tautomers of the alloxazines with two hydrogen-bearing nitrogen atoms in one ring. Lotsch and her colleagues were the first to integrate this alloxazine chromophore in a COF to combine the advantages of organic photocatalysis with heterogenic applicability in the lab.
COFs, which are porous, crystalline networks, are usually made by the polycondensation of long, rigid, multifunctional organic molecules. To prepare the alloxazine unit as a building block for COFs, the researchers attached two formylphenyl units opposite to each other to the alloxazine benzyl ring to react them with the three amino groups of an aminophenyl-substituted triazine. In this way, dye and triazine condensed to form a crystalline solid with nanometer-wide hexagonal pores. The walls of these pores carried the alloxazine chromophore.
Heterogeneous, All-Organic Photoredox Catalysis
This integrated alloxazine unit could neither tautomerize nor aggregate because its nitrogen-bound hydrogens were replaced by ethyl groups. The researchers reported that this condensation synthesis block indeed produced a stable and active photoredox catalyst.
They studied the oxidation reaction of an aromatic alcohol—methoxy-substituted benzyl alcohol—in oxygenated watery solution under irradiation with blue light. The yield was not particularly impressive—44 to 79 % under various conditions tested—but the reaction was remarkably selective and produced mainly 4-methoxybenzaldehyde, with only the respective benzoic acid “as a minor side-product”, said the researchers.
Apart from providing stability, the integration of the chromophore in the COF also reduced the energy required to activate the catalyst. The researchers noted that in the crystal, the alloxazine absorption band broadened, making reactions with longer-wavelength green light irradiation possible.
Selective Reaction with Limited Scope
The photoredox mechanism put forward by the team involves a superoxide radical for alcohol oxidation. It is generated when the light-activated COF catalyst withdraws an electron from the alcohol and transfers it to molecular oxygen. Then, it abstracts a proton from the oxidized alcohol to generate the aldehyde.
This mechanism leads to a selective reaction (as no hydroxyl radical or singlet oxygen is involved), but it also limits the substrate scope. As the oxidation potential of the alcohol substrates must fit within the valence band energy of the photocatalyst, the researchers were unable to convert less reactive substrates such as furfuryl alcohol or nitro- and unsubstituted benzylic alcohols. In contrast, other selective non-alcoholic aerobic oxidations took place, such as sulfoxidations and selective C–H oxidations.
The study shows that COF-based photocatalysis combines many valuable properties: biologically inspired specific functionality, a porous network suitable for heterogeneous catalysis, and the all-organic, metal-free nature of the catalyst. The researchers propose the underlying synthetic chemistry will make it possible to create a variety of photocatalysts with interesting chromophores.
- A flavin-inspired covalent organic framework for photocatalytic alcohol oxidation,
Stefan Trenker, Lars Grunenberg,Tanmay Banerjee, Gökcen Savasci, Laura M. Poller, Katharina I. M. Muggli, Frederik Haase, Christian Ochsenfeld, Bettina V. Lotsch,
Chem. Sci. 2021.
https://doi.org/10.1039/d1sc04143f