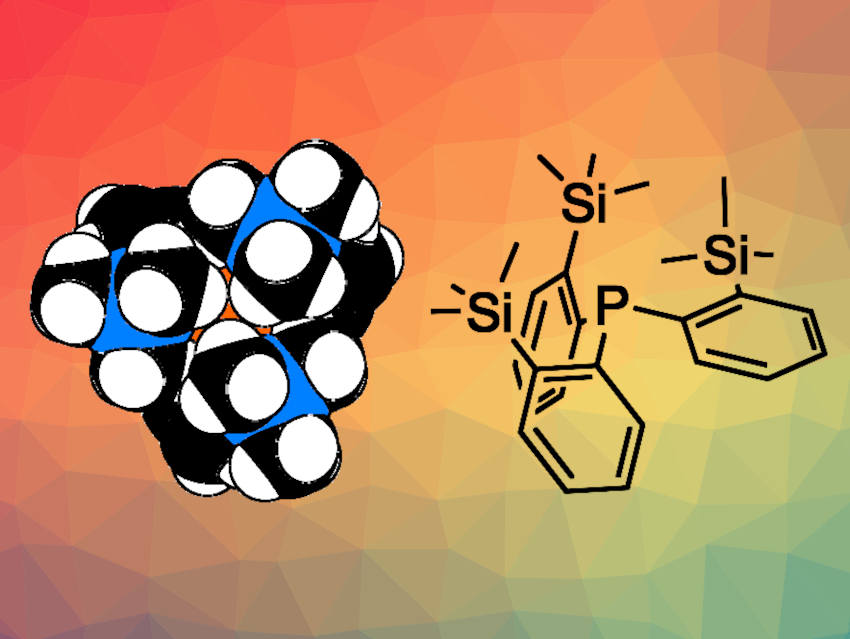
Phosphines represent a particularly popular class of ligands in coordination chemistry. Their properties are highly adjustable: The steric requirements and electronic properties of these ligands can be tuned independently.
Ulli Englert and colleagues, RWTH Aachen University, Germany, have synthesized the sterically crowded phosphine tris(2-(trimethylsilyl)phenyl)phosphine (P(o-TMSC6H4)3, pictured). The team first prepared tris(2-bromophenyl)phosphine from 1-bromo-2-iodobenzene and PCl3. The resulting intermediate was subjected to a triple bromine/lithium exchange and then reacted with trimethylsilylchloride (TMSCl) to give the desired bulky phosphine.
The bulky ortho-substituents in the molecule shield the phosphorus atom and restrict reactivity. Typical reactions involving unencumbered phosphines are impeded for this phosphine—for example, P(o-TMSC6H4)3 does not react with oxygen from the air. The coordination to Au(I) is one of the few reactions this crowded phosphine can still take part in. Complexation was unsuccessful with a range of other metal cations. However, the steric shielding also provides high stability, which allowed the researchers to study the phosphine radical cation. This species could serve as a model compound for the radical cations of simpler, commercially relevant triarylphosphines.
- Sterically Crowded Tris(2‐(trimethylsilyl)phenyl)phosphine – Is it Still a Ligand?,
Hans Gildenast, Felix Garg, Ulli Englert,
Chem. Eur. J. 2021.
https://doi.org/10.1002/chem.202103555