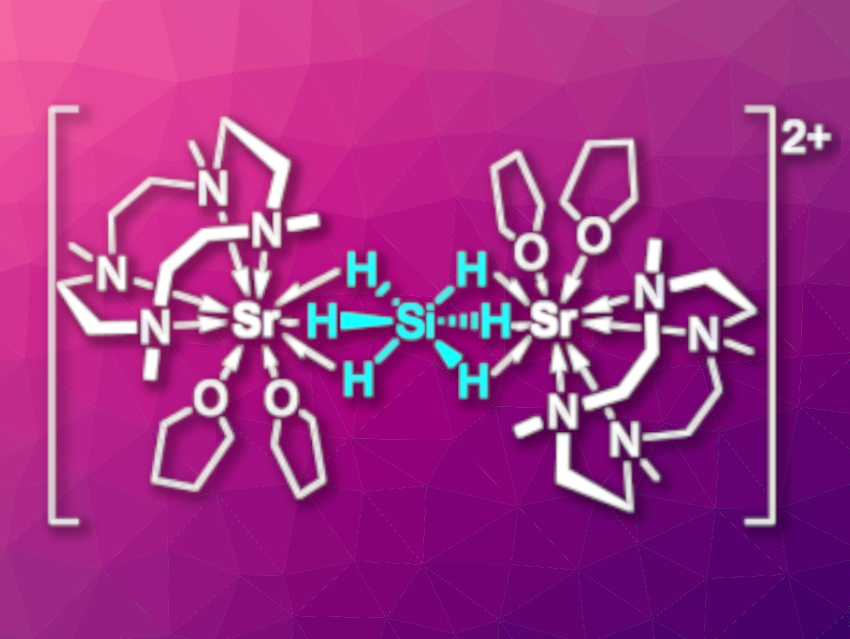
Monosilane SiH4 is an industrial gas used for the production of silicon semiconductors. In contrast to its carbon analogue methane, SiH4 can expand its coordination sphere, forming, e.g., the hypervalent six-coordinate anion [SiH6]2–. However, the isolation of [SiH6]2– in the condensed phase is challenging.
Jun Okuda, RWTH Aachen University, Germany, and colleagues have used a cyclen-derived macrocycle that can stabilize reactive metal hydrides of electropositive metals to “trap” [SiH6]2– in a dinuclear strontium complex (pictured). The complex was prepared via a reaction of the cationic benzyl complex [(Me4TACD)Sr(CH2Ph)][B(C6H3-3,5-Me2)4] (Me4TACD = 1,4,7,10-tetramethyltetraazacyclododecane) with phenylsilane in tetrahydrofuran (THF).
When the complex decomposes, it releases H2 to give a strontium complex with a silanide unit (SiH3–). An improved understanding of the reductive elimination of H2 from hydrogen-rich silicon compounds could be useful, e.g., in the context of H2 uptake and release in the hydrogen storage material K4Si4/KSiH3. In reactions of the complex with a weak Brønsted acid, CO2, or cyclooctatetraene, SiH4 was released. This suggests that the synthesized complex can be considered an adduct of monosilane with two strontium hydride cations.
- Formation and Reactivity of a Hexahydridosilicate [SiH6]2− Coordinated by a Macrocycle‐Supported Strontium Cation,
Jun Okuda, Thomas Höllerhage, Priyabrata Ghana, Thomas P. Spaniol, Ambre Carpentier, Laurent Maron, Ulli Englert,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202115379