Food is the product of value-added chains. Vegetables have been sown or planted, watered, harvested, and transported. A tomato sauce has been processed from tomatoes and several other ingredients with their own production chain. To produce bread, the grain must be planted and harvested, a dough prepared and baked. Regardless of all the efforts, resources, energy, and water spent for food production, millions of tons of food are wasted in western countries. For instance, in Germany, about 80 kg of food per person is discarded every year [1]. More than 50 % of this waste is caused by private households.
Today, children and young people help to decide on the food bought for their families, and they also have a significant part in food-waste production. However, young people, in particular, are often engaged in activities against food waste, like food-sharing projects or dumpster diving. Thus, it appeared reasonable to target them in a project aimed to learn more about the natural science of food (see Fig. 1).
Sensitivity to the problem has grown over the last few years. Many initiatives try to modify our behavior and give advice on how to avoid food waste. But as chemists and especially food chemists, we look at food as something that changes according to natural laws. Knowing about this, we can better understand food and treat it properly.
 |
|
Figure 1. Use, not waste! Better understanding of food by natural science (graphic by Tobias Tank, but-design.de [2]). |
The Agnes Pockels Student Laboratory
At the Technische Universität (TU) Braunschweig, Germany, a laboratory for students has been operated for about 20 years now, the Agnes Pockels Student Lab [3,4]. It aims at raising and supporting an interest in natural science early by offering hands-on experiments in a chemistry lab (see Fig. 2). The objectives are related to everyday life, e.g., to water, polymer materials, food, cosmetics, and to socially-relevant challenges with relation to natural sciences, for example, energy transformation and storage, sustainability, or climate change [5]. Thus, it was natural to develop experiments dealing with the problem of food waste. This project was funded by the Deutsche Bundesstiftung Umwelt (DBU, German Federal Environmental Foundation) [6].
 |
|
Figure 2. Students performing experiments in the Agnes Pockels Lab at TU Braunschweig. |
Food from the Perspective of a Chemist
What is food from a chemist’s perspective? Mostly, it is an artful composition of many different components, non-polar and polar materials, organic and inorganic ones, high- and low-molecular-weight compounds—at amounts spanning several orders of magnitude, from bulk to trace elements.
However, food is more than the sum of its ingredients. Its taste is strongly affected by its physical structure. It strongly depends on how intrinsically immiscible constituents are brought in contact or emulsified (e.g., in chocolate or ice cream), whether they are elastic, brittle, or slimy. Our palate works like a rheometer when shearing the food in the mouth.
Food is never in a thermodynamic equilibrium. It changes over time, but at very different rates: over the years or within hours. These changes alter the quality, not always in a negative way—e.g., when we think of ripening and flavor development. Still, the changes can cause spoilage in the end, including inedibility and health risks.
The changes comprise physical processes like the separation of emulsions or the collapse of foams, chemical reactions like fat oxidation or enzymatic/non-enzymatic browning, as well as (micro)biological processes where bacteria or fungi metabolize food for their own growth, often accompanied by the production of toxins. Examples are molds on citrus fruit and salmonella on eggs or chicken. But what are the parameters that influence the rate of these changes?
Water, Temperature, and pH
From experience, we know that dry food is more “durable”, while wet food like fresh meat or fish is more perishable. Furthermore, we know that cooling or freezing is a common measure to extend the life of foods. Thus, water and temperature are two important parameters (see Fig. 3).
Most vegetables and fruit contain about 95 % of water, a surprisingly huge amount. However, it is not only the “original” content of water that counts. Dry food still contains about 7–12 % water. After subjecting dry foods to an ambient atmosphere, the students measure humidity uptake, rather than loss of the residual water. On the contrary, drops of pure water would evaporate with time. So, what makes the difference?
Water is bound in food mainly in three ways: (1) by physisorption on surfaces: a monomolecular layer of water is bound so strongly that it will not be released; (2) by capillary forces: especially in meat, water is retained in capillaries. The smaller the radius, the stronger the force, and (3) by osmotic pressure: this phenomenon is used in conservation methods such as salting or preparing jam with sugar. When used at a higher molar concentration than in the cells, the dissolved ions or molecules draw water out of the cells, and thus, reduce the amount of water available for spoilage.
Students can study these effects with slices of potato, treated with deionized water or 10 % NaCl solution, making the slice firm by swelling or soft by shrinking. All the phenomena described above reduce the vapor pressure of the water in food. The vapor pressure above food related to that of pure water at a certain temperature defines the water activity aw. This is the crucial parameter for food stability. Bacterial growth requires an aw above 0.9, while fungi remain active up to an aw of 0.7. Bound water is not available for microbial growth and the dissolution and transport of reactants. This affects chemical and enzymatic reactions, as well.
.jpg) |
|
Figure 3. Impact of temperature and water activity on food. (MO = microorganisms, i.e., bacteria, yeast, fungi) |
The temperature can also play a role in the availability of water. Freezing immobilizes water, but at the same time, it reduces the rate of reactions due to kinetic laws. This is the crucial aspect of cooling food. The impact of reduced temperature is widely underestimated—not only the fridge, but even keeping leftover meals in a cool basement instead of on the stove extends its lifetime significantly.
When we go, however, into the other direction, to higher temperatures, we reach a range at which proteins are denatured. This is also important for conservation, since the metabolism and growth of microorganisms are based on active enzymes, which are proteins. These proteins catalyze biochemical reactions by acting as a scaffold that fixes the substrate in the perfect positions and comprising the “instruments” to perform the chemical transformation. At higher temperatures, i.e., higher energies, the scaffold becomes unstable and breaks down.
Therefore, higher temperatures are used for pasteurization and sterilization and for the deactivation of food enzymes. In our lab, students experience this by measuring the activity of yeast by its CO2 production at various temperatures and by checking whether reactivation is possible (see Fig. 4). Further aspects that are treated in the project are the sublimation of water (freezer burn) and crystal growth related to the cooling rate.
 |
|
Figure 4. CO2 production by yeast as a measure of its growth activity at various temperatures. In a second experiment, yeast held at 5 °C and 75 °C is incubated at 40 °C. |
Besides a temperature optimum for the growth of microorganisms or the activity of enzymes, there is also a pH optimum. Therefore, acid is can be used for the preservation of food, e.g., in mixed pickles or sauerkraut.
Light, Air, and Contact Materials
Less obvious than the above-mentioned parameters are the influences of light and air on the changes in food. Lipids, especially oils with many unsaturated fatty acids, are sensitive to oxidation and photooxidation, promoted even by traces of heavy metal ions such as Fe2+/Fe3+ or Cu+/Cu2+. Here, another aspect comes into play: the contamination by migration from kitchen implements and packages. To a certain degree, the spoilage of oil-rich food like nuts and related drupes is mostly recognized by a stale or musty smell.
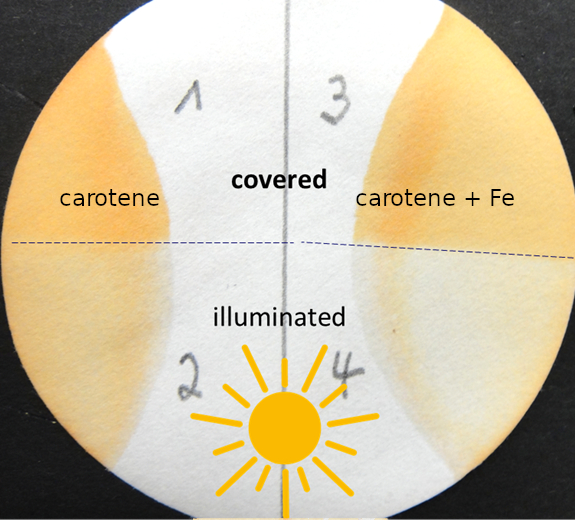
The experiments in the student lab must allow the recognition of changes in food without analytical instruments and within the time of a lab visit. For example, the highly unsaturated, lipid-associated carotenoids are sensitive to photooxidation. This can be studied by the students in the lab. A filter paper soaked with carotene is exposed to light, while half of it is kept in the dark. After a few minutes, the loss in orange color on the irradiated part can be recognized. This is even more pronounced if the carotene was doped with an iron salt (see Fig. 5).
 |
|
Figure 5. Illumination of carotene on a filter paper without (2) and with (4) addition of an iron salt. (1) and (3) are the controls, covered during irradiation. |
In another experiment, milk is exposed to light. Riboflavin (vitamin B2) promotes the formation of dimethyl disulfide from the amino acid methionine, easily recognized by its smell because of its very low threshold value. The fluorescence of riboflavin under UV light also decreases when exposed to light for a while. From these experiments, students learn why milk and oils should be stored protected against light.
Is Change Always Bad?
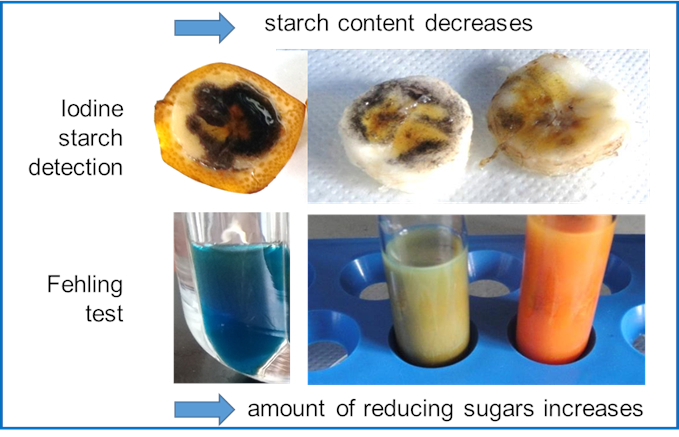
(Nearly) every process passes through an optimum—This statement also applies to food. Some fruits and vegetables are harvested unripe, e.g., bananas. Green bananas contain a lot of starch. During ripening, the starch, as a non-osmotic storage form of photosynthetically produced glucose, is depolymerized to sugar. The banana becomes sweet and softer and develops aroma compounds. Wherever the optimum of taste is classified by personal opinion, the rate of this change depends on temperature.
Students can follow the ripening process by detecting the vanishing starch with iodine and the increasing amounts of mono- and oligosaccharides by the Fehling test (see Fig. 6). They can compare bananas from one stem stored at various temperatures, concluding that it might be reasonable to store not all bananas at the same temperature.
 |
|
Figure 6. Following the ripening of bananas by the depolymerization of starch. |
Conclusion
Based on experiments with various foods, students recognize how the availability of active water, the rate and type of changes, and the activity of enzymes and microorganisms depend on structure and composition, temperature, pH, light, and air. In further experiments, they study the transmissibility of various materials for air and humidity, and the contamination by metal traces. Students were motivated and engaged in the lab work due to the connection of the experiments to their daily lives. Interviews before and after the lab visit showed increased sensitivity for food waste and the intention to change one’s own behavior. Experiments and more details can be found on the webpage of the Agnes Pockels Student Laboratory [2,5].
References
[1] Lebensmittelabfälle in Deutschland: Aktelle Studie über Höhe der Lebensmittelabfälle nach Sektoren (in German), Bundesministerium für Ernährung und Landwirtschaft, www.bmel.de. (accessed January 20, 2022)
[2] Petra Mischnick, Ilka Deusing-Gottschalk, Verwenden, nicht verschwenden! Durch Experimentieren Lebensmittelversteher*in werden, TU Braunschweig, Germany, 2021. (accessed January 20, 2022)
[3] P. Mischnick, B. Faustmann, The Agnes-Pockels-Schülerlabor – An example of how non-formal laboratories can contribute to education for sustainability, in I. Eilks, S. Markic, B. Ralle (Eds.), Science Education Research and Education for Sustainable Development, Shaker Verlag 2014, 215–218. ISBN: 978-3-8440-3150-8
[4] P. Mischnick, Learning chemistry—the Agnes-Pockels-Student-Laboratory at the Technical University of Braunschweig, Germany, Anal. Bioanal. Chem. 2011, 400, 1533–1535. https://doi.org/10.1007/s00216-011-4914-6
[5] Agnes Pockels Laboratory, www.tu-braunschweig.de. (accessed January 20, 2022)
[6] Experimentieren, Verstehen, Verhalten verändern: Naturwissenschaftliche Grundlagen zur Ernährungsbildung in Schule, Schülerlabor und Museen, Deutsche Bundesstiftung Umwelt (Az 34539/01-44), www.dbu.de. (accessed January 20, 2022)

Authors
 Prof. Dr. Petra Mischnick
Prof. Dr. Petra Mischnick
Technische Unversität Braunschweig, Institute of Food Chemistry and Agnes Pockels Student Laboratory
E-mail: [email protected]
Petra Mischnick works in the field of polysaccharide and polysaccharide derivatives. Her research focuses on the functionalization and structure analysis of carbohydrates by mass spectrometry. In 2002, she founded the Agnes Pockels Student Laboratory to promote the interest and knowledge of children, especially girls, in natural sciences. Experiments are related to everyday life and socially relevant challenges like sustainability, energy, climate change, and food waste. In 2021, her work was honored by the Hildegard-Hamm-Brücher award for equal opportunities in chemistry by the German Chemical Society (GDCh).

Dr. Ilka Deusing-Gottschalk
Agnes Pockels Student Laboratory
Technische Universität Braunschweig
E-mail: [email protected]
Ilka Deusing-Gottschalk is a state-certified food chemist and received her Ph.D. in 1996 from the Technische Universität of Braunschweig, Germany, in the field of carbohydrate chemistry. After holding different positions in the sugar industry since 1994, she joined the Agnes Pockels Student Laboratory in 2007. At first, she mainly operated workshops for girls and boys to promote their interest in the natural sciences. Furthermore, she developed concepts and experiments for school labs in various subjects, e.g., (bio)polymers or energy. Currently, she operates the project “Reducing the waste of food with natural sciences” funded by the Deutsche Bundesstiftung Umwelt (DBU, German Federal Environmental Foundation).
Also of Interest
 Video: Overcoming Well-Meaning Discouragement
Video: Overcoming Well-Meaning Discouragement
Petra Mischnick about her motivation to advocate for equality, the importane of role models and teaching chemistry from early on