Desalination removes salts of various metals and impurities from seawater. It is used to obtain fresh water for human consumption and for domestic/industrial use. Extracted salts and other metal compounds present in the desalination mix form a hyper-saline mixture commonly referred to as brine. This waste by-product contains mainly sodium chloride (NaCl) and water. Its composition depends on the type of desalination process and the area where the desalination takes place.
The economic growth and global competitiveness of the European Union require the availability of vital raw materials of strategic relevance—critical raw materials or CRMs—to strengthen long-term industrial leadership. The extraction of minerals from hypersaline brines generated as by-products of seawater desalination plants has attracted the interest of scientists.
About 18,200 desalination plants operate worldwide with a global cumulative capacity near 90 million m3/day [1]. This capacity is expected to grow at an increasingly rapid pace in the near future because of the water shortage crisis around the world and increasing water demand [2].
Brine generated in seawater desalination (SWD) is currently discharged into the sea, generating negative environmental impacts and posing a potential danger for marine ecosystems due to its high salinity. Furthermore, if the brine is discharged, the energy used for its production will be lost as well as the benefit of using it as a potential source of metals and critical metals.
For the recovery of the brine, however, a lot of research is required. In particular, a European project with the acronym SEA4VALUE is working on how to extract metals and critical metals while minimizing the negative environmental impact. The vision of the project is to create a multimineral and modular process to separate, concentrate, and crystallize molybdenum, magnesium, scandium, vanadium, gallium, boron, indium, lithium, and rubidium in seawater desalination plants. A first challenge is the removal of the large amounts of calcium from brine. Another issue is the amount of energy required.
1. What Are Critical Metals and Why Is Brine a Potential Source?
The scarcity of some elements is leading to new strategies to meet the increasing industrial demand. From a geochemical view, most of the elements, except Al, Si, Fe, Ca, Mg, Na, and K, are scarce in the sense that they are mined from medium to high-grade ores that risk depletion [3].
The European Union list of critical metals (see Tab. 1) highlights the policy interest in these scarce elements [4]. The reasons behind the growing concern over the supply of the metals are their increased societal use, the depletion of the conventional mined resources, and the political and economic stability of some major producers.
|
Table 1. Critical Raw Materials list from the European Union (2020) [4]. |
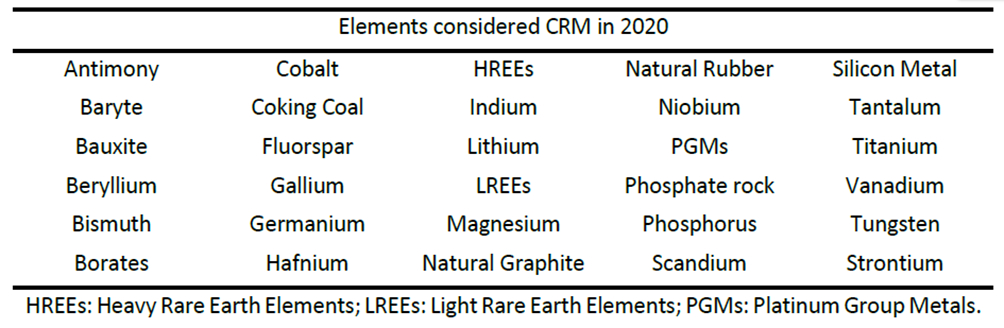 |
Strategies used to alleviate this problem have been:
i) the exploitation of low-quality primary ores,
ii) “recovery” from unconventional sources like industrial waste streams considered as secondary resources, and
iii) material substitution by a more easily available metal or element or reduction, where the amount of a metal or element needed is diminished by a co-substitution principle.
According to Zimmermann et al. [5], 30 % of all EU-classified critical materials are wasted and many of them have dissipation rates higher than 50 %.
Seawater reverse osmosis (SWRO) desalination brines, which are the majority of the SWD brines [2], are waste streams generated in the production of drinking water that contain the source components in higher concentrations than the initial seawater by typically a factor of two (see Tab. 2) [6].
|
Table 2. Average values of elements present in seawater reverse osmosis concentrates [6]. |
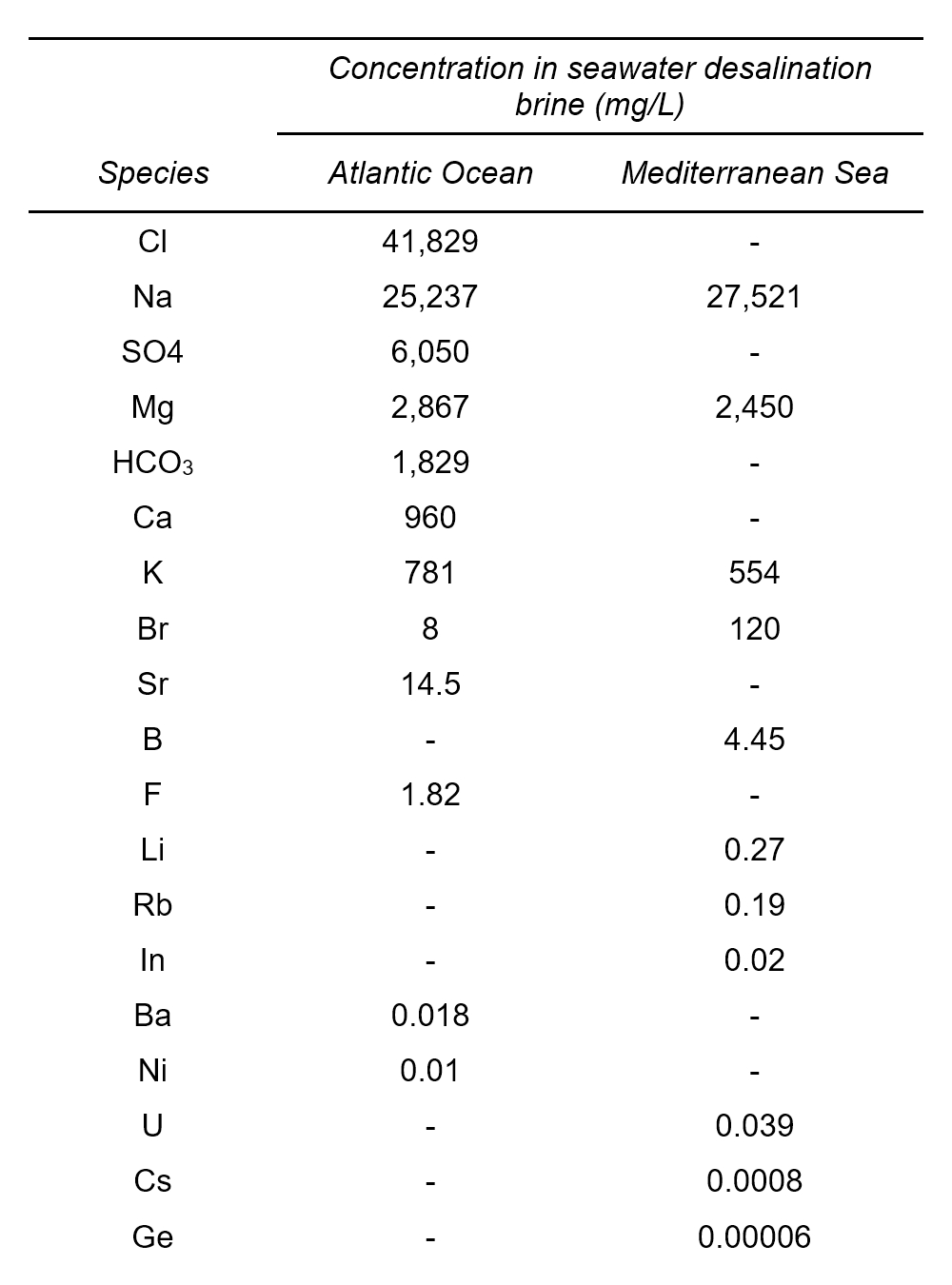 |
The predicted worldwide desalination capacity is quite variable. According to the sources of the references [1] and [7], it can be foreseen to be in the range of 90–150 million m3 per day, and it is expected to continue growing according to climate change predictions [7].
The SEA4VALUE project will investigate the feasibility of recovering Li, B, Mg, Sc, V, Ga, Rb, Mo, and In from brines generated in SWD plants. To achieve this, innovative technologies will be developed, including advanced nanofiltration, membrane crystallization, advanced multi-effect distillation, and selective processes such as adsorption and solvent extraction (see Fig. 1).
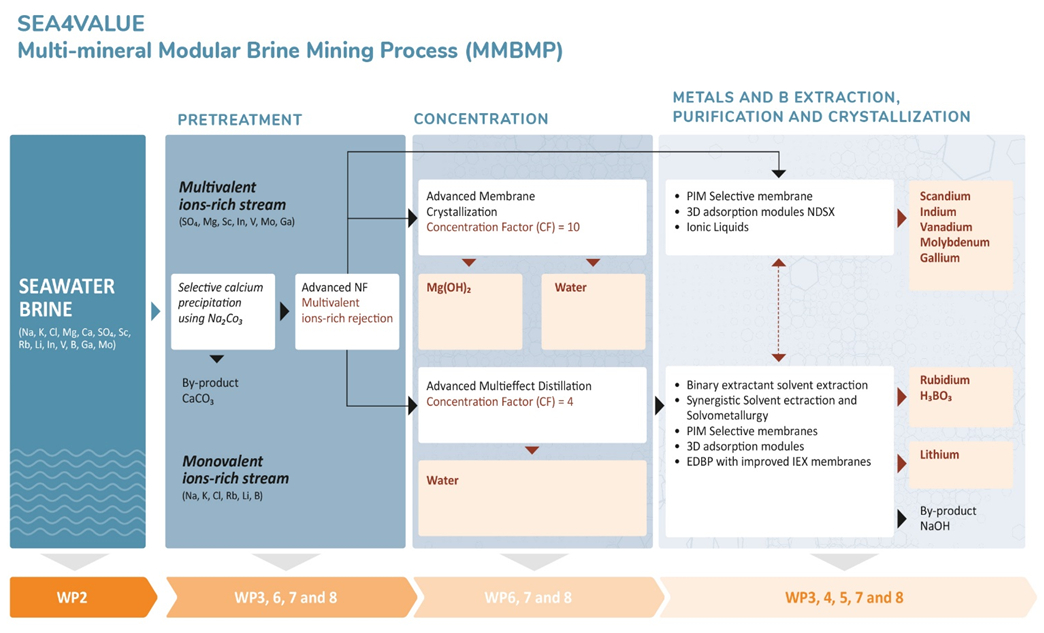 |
|
Figure 1. Simplified scheme of process steps for metals recovery predicted in the SEA4VALUE project. |
2. Benefits of Ca Removal from Brine for Metals Recovery
One of the key limitations associated with the recovery of critical elements, which are present at trace levels, from reverse osmosis (RO) brine is the necessity to remove the main major elements, specifically divalent elements such as Ca(II) and Mg(II). Since Mg is one of the target elements in SEA4VALUE, it should be selectively separated from Ca, which is a challenge.
Values of Ca(II) concentrations in SWRO desalination brines are in the range of 700–900 mg(Ca)/L. There is a double need for Ca(II) removal:
i) It helps to prevent scale formation in most of the needed separation stages to recover the different targeted trace elements.
ii) It helps to improve the selectivity factors of the trace elements, if they are present as major components compared to the remaining Ca(II).
As stated, calcium removal is a required pre-treatment in the SEA4VALUE project. The removal of Ca(II), in the form of calcium carbonate precipitation from brines, was critically reviewed by Bond and Veerapaneni [8] along with desalination processes. The use of fluidized-bed crystallizers for the production of calcium carbonate pellets [9], the influence of antiscalants used in RO seawater desalination processes, and the presence of impurities (e.g., other metal ions) on the calcium carbonate precipitation were the main aspects to be considered [10].
While the concentration of calcium in seawater and desalination concentrate is relatively high, the extraction of a saleable calcium product from seawater at an industrial level has garnered very little attention, with only one notable exception at a brackish water desalination facility in Southern California, USA [9].
It should be mentioned that the extraction of Ca(II) from seawater or RO concentrate in the form of calcium sulfate has not received significant attention due to the low price of commercial-grade gypsum. For that reason, and as well as considering the higher solubility of this salt compared to calcium carbonate, gypsum precipitation was discarded in the SEA4VALUE project [11,12]. Other options such as the use of ion-exchange should be discarded as it is not possible to remove Ca(II) and Mg(II) selectively [13]. All these considerations pushed the challenge of selective Ca removal as a pretreatment before the other process steps as reported in Figure 1 and described in the following.
3. Separate Removal of Calcium and Magnesium
Calcium and magnesium are two of the most abundant electrolytes in seawater, and their separate recovery can be useful for various applications. In the SEA4VALUE project, a first result is the calcium recovery as calcium carbonate while magnesium is predicted to be recovered as magnesium hydroxide in successive process steps. Each one of these elements has several uses [14,15].
To enable a selective separation, the different reactivity of Ca and Mg due to their different hydration was used. Magnesium ions are more hydrated than calcium ions [16], which leads to a lower ability to combine with negative ions, such as the carbonate ion. This leads to easier calcium carbonate precipitation compared with magnesium carbonate [17,18]. However, magnesium in brine is five times more concentrated than calcium, inducing a reduced growth rate of calcite (a crystalline form of calcium carbonate) as observed by several researchers [19–21]. As a result of the higher Mg concentration compared to the Ca concentration, magnesium can hinder calcite crystallization, but this depends on operating conditions, as shown below.
Experiments using solutions simulating an average brine were carried out at different pH, temperature, ionic strength, and carbonate/calcium ion molar ratios (R) to try to increase calcium precipitation by minimizing magnesium removal (see [18] for detailed results). A particular parameter of interest was the temperature, as its increase allowed to increase the growth rate of calcite, as shown in Figure 2 using a solution containing Ca and Mg cations.
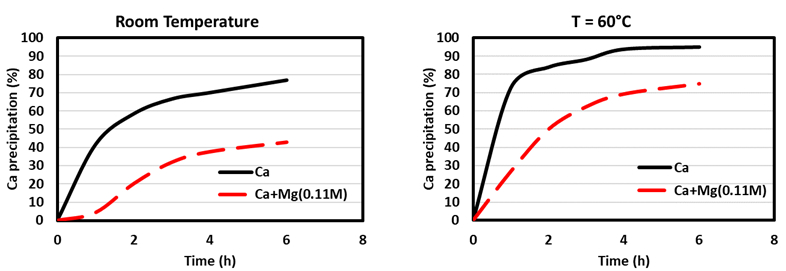 |
|
Figure 2. Effect of Mg(II) on Ca(II) precipitation at (a) room temperature and (b) 60 °C (elaborated from [18]). |
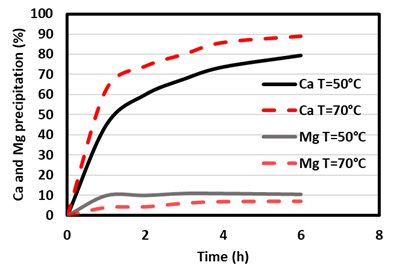
It can be seen that at room temperature, the effect of Mg on Ca precipitation is more pronounced than that at a higher temperature. Furthermore, at higher temperatures, an increase of Ca precipitation is observed. Subsequently, a simulated brine (SB) solution gave a Ca precipitation of around 85 %, while the Mg co-precipitation was around 7 % (see Fig. 3).
 |
|
Figure 3. Effect of the temperature on Ca and Mg precipitation in the SB solution (pHNaHCO3 = 9.0 and R = 3; elaborated from [18]). |
4. How Integration between Experiments and Modeling Can Help Research
Chemical modeling is a powerful tool used to simulate chemical processes from a thermodynamic perspective. Modeling can be used to predict calcium precipitation in different conditions, reducing the cost of experiments by defining optimal conditions. However, the simulation of processes at high ionic strength requires specific models. Different codes and software can be used for modeling processes at high ionic strength, e.g., OLI and PhreeqC, both of which are used in SEA4VALUE.
A thermodynamic model in the form of PhreeqC code that is supported in the computation of the activity coefficients and the mineral saturation indexes (SI) has been used to analyze the precipitation of calcium carbonate [22]. Due to the high salinity of the solutions, the Pitzer database was used, and it was necessary to extend it to elements conventionally neglected for their low contents in brine (mg/kg to μg/kg) that are now considered in the identification of alternative mineral resources.
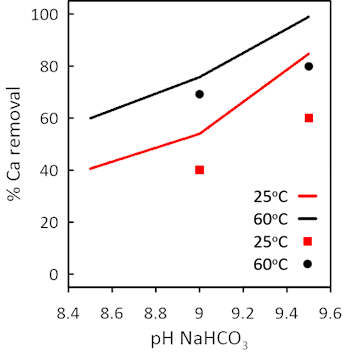
Using the initial composition of the RO seawater brine and considering the information of the experimental precipitation trials, the variation of major and minor solutes concentrations in both the brine and solid phases was simulated with the PhreeqC (v3.6.2) numerical code [23]. Finding the Ca removal in percent as a function of the bicarbonate concentration was the main objective of the modeling stage, and the validation of the modeling effort was carried out using the experimental data generated in the precipitation reactions.
As can be seen in Figure 4, the experimental values are in good agreement with those predicted by PhreeqC. The numerical code can then be used to optimize the removal of Ca(II) in terms of levels of total carbonate and pH to avoid the potential co-precipitation of Mg(II).
 |
|
Figure 4. Comparison of modeling (solid lines) and experimental data (square and lozenge points) in Ca(II) precipitation as a function of the initial pH (elaborated from [18]). |
5. How Nanofiltration Can Contribute to Increased Water- and Trace-Element Recovery
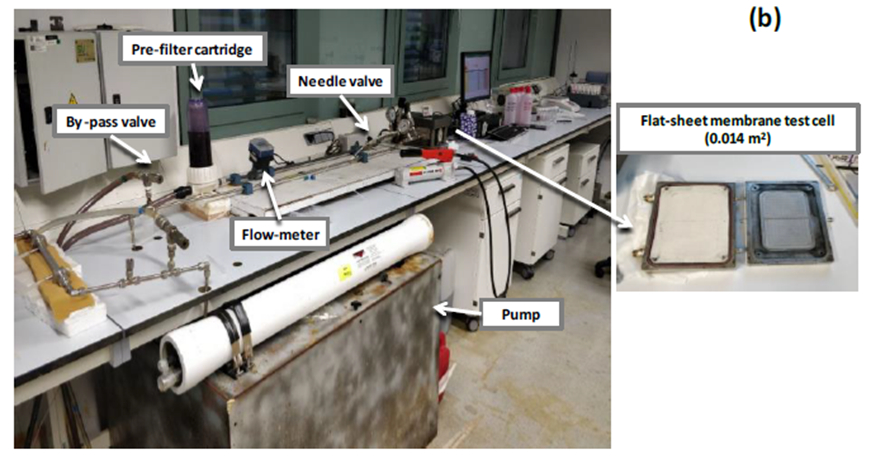
After the removal of Ca(II), the rich Mg(II) brine, which also contains targeted trace elements to be recovered, could be subjected to a separation stage based on the use of nanofiltration (NF) membranes (see Fig. 5). NF membranes based on an active layer of semiaromatic polyamides (e.g., from DuPont) have shown high rejection values (>80 %) for multivalent ions (e.g., Ca(II), Mg(II), Sr(II), and sulfates) and low rejection values for monovalent ions (Na, K, Cl) and most of the targeted trace elements to be recovered. The use of NF can be seen as a pre-purification stage of the brine to remove those ions affecting the subsequent process treatment stages.
 |
|
Figure 5. Experimental set-up with nanofiltration membrane for the evaluation of brine pre-purification; (b) detail of a flat-sheet membrane test cell. |
Nanofiltration has been postulated by Telzhensky et al. [24] and Reig et al. [25] as a brine pretreatment step to produce rich Mg(II) and Ca(II) concentrates or Mg(OH)2(s). The rejection of polyvalent ions was tested with a new family of NF membranes, where the active layer has been modified using a layer-by-layer (LBL) deposition technology. Typically, in a LBL deposition high-molecular-weight poly-electrolytes are adsorbed into the active layer of a membrane.
The rejection generally increased with pressure in the range studied (from 8 up to 20 bar). Undesired NaCl rejection also increased, reaching a maximum of 12 % at 20 bar. However, divalent ions were more easily rejected than NaCl, so the NF objective was achieved. The rejection of major ions became stable with the increase of pressure, reaching a plateau at pressures of 18 bar. The rejection order with SWD-RO brine at 20 bar was Al(III) > S(VI) > Mg(II) > Sr(II) > Cu(II) > Ca(II) > Ni(II) > NaCl > K(I).
NF270 is a negatively charged membrane, so higher rejections were observed for anions, with the exception of Al(III). As a trivalent ion, the dielectric exclusion mechanism explains its higher rejection than divalent ions or monovalent ones. Dielectric exclusion is caused by the interactions of ions with the bound electric charges induced by ions at interfaces between media of different dielectric constants. It is considered one of the mechanisms of nanofiltration. The presence of a fixed electric charge of noticeable magnitude in the membrane surface may cause a strong screening of dielectric exclusion., i.e., the higher the charge of the ion, the higher its rejection.
The initial concentration of an ion plays a significant role in its rejection degree. When highly concentrated brines are used, a screen phenomenon (selective crossing of ions through the membrane) happens on the active membrane surface layer and the Donnan effect (non-symmetric distribution of charged particles across the two sides of the membrane) is minimized. Counterions screen the charge of the membrane’s surface layer and, in consequence, sieving becomes the dominant mechanism for ionic species rejection. Ions with a smaller hydrated radius, such as Na(I) and K(I), are less easily rejected from the brine than the other ions, such as multivalent ions.
6. How Calcium Removal from Brine Can Be Used to Develop a Process with Near-Zero Liquid Discharge
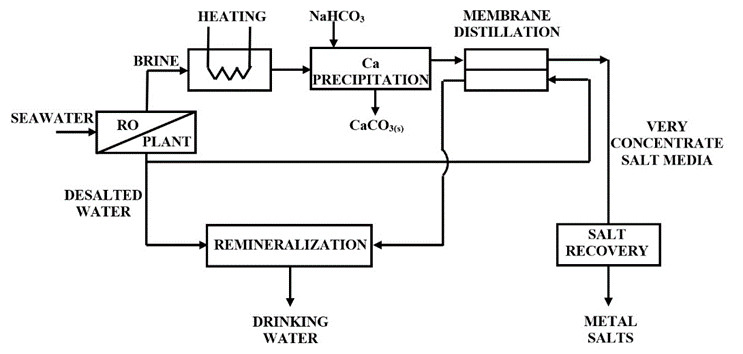
Since a brine temperature of 60–70 °C is required for calcium removal, the brine discharged from the reverse osmosis system should be heated to this temperature range. This should be done, for example, with the help of solar collectors to increase the sustainability of the process. Ca is then removed by precipitation at controlled pH.
Since a membrane distillation (MD) operation is based on the temperature difference between a warm and a cold stream, a subsequent stage based on MD could take advantage of the high temperature.
The low Ca content reduces the scale formation on the membrane usually associated with calcium-based minerals. Thus, the water flux will not decrease significantly, and the operational and economic efficiency of membrane distillation is improved. Consequently, the brine valorization process can be enhanced by developing a process characterized by near-zero liquid discharge (NZLD), where almost all components contained in the brine (water and salts) are recovered.
From the MD unit, water is recovered and, after remineralization, drinking water is obtained. The concentrated mixture of the various elements which were initially present in the seawater at very low concentration is sent to salt recovery (see Fig. 6). In this mixture, Mg(II) is the main component, together with NaCl and minor elements.
 |
|
Figure 6. Conceptual scheme of brine valorization after prior Ca removal for approaching NZLD [18]. |
7. Conclusion and Outlook
The research on technologies and processes that can take advantage of brine as a metal source, is fully open and, to our knowledge, the approach of selective calcium precipitation as described here has been studied only by Wang et al. [26], who obtained comparable results working at a temperature (85 °C) higher than the ones we investigated (up to 70 °C). The processing scheme (see Fig. 6) is an example of a clean process that was possible to design after collecting information to deeply understand the fundamentals of one of the involved chemical processes.
The study of the chemical aspects of calcium removal from brine integrates theoretical aspects, i.e., brine modeling with geochemical equilibrium codes such as PhreeqC, and experimental efforts. The use of sodium hydrogen carbonate reduced Mg loss during the precipitation of CaCO3(s) because the pH was controlled at a value below that of magnesium hydroxide precipitation.
The overall results (pH 9.0, temperature of 60–70 °C, molar ratio HCO3–/Ca = 3, Ca removal efficiency greater than 90 % with a Mg loss around 7 %) are of potential interest to design an NZLD process for brine valorization treatment.
A bottleneck of the setup shown in Figure 6 is the necessity to heat the brine to 60–70 °C. A large amount of energy is required considering both the high brine flow-rate and the necessary temperature during the calcium carbonate precipitation process.
Another approach that may be more sustainable from an energy standpoint, which was also explored as part of the SEA4VALUE project, is to operate at a temperature closer to the ambient one. This is easier to control and maintain. Tests on a lab-pilot plant operating on site are planned, and the results should be available within a few years.
References
[1] International Desalination Association, idadesal.org. (accessed on April 29, 2022)
[2] M. Badruzzaman et al., Selection of pretreatment technologies for seawater reverse osmosis plants. A review, Desalination 2019, 449, 78–91. https://doi.org/10.1016/j.desal.2018.10.006
[3] M. L. Söderman et al., in Syst. Perspect. Electromobility, B. A. Sandén (Ed.), Chalmers University of Technology, Gothenburg, Sweden, 2014, 76–89.
[4] European Commission, Study on the EU’s list of Critical Raw Materials (2020): Final Report, 2020. https://doi.org/10.2873/11619
[5] T. Zimmermann, Uncovering the fate of critical metals: tracking dissipative losses along the product life cycle, J. Ind. Ecol. 2017, 21, 11981211. https://doi.org/10.1111/jiec.12492
[6] P. Loganathan et al., Mining valuable minerals from seawater: A critical review, Environ. Sci. Water Res. Technol. 2017, 3, 37–53. https://doi.org/10.1039/c6ew00268d
[7] E. Jones et al., The state of desalination and brine production: a global outlook, Sci. Total Environ. 2019, 657, 1343–1356. https://doi.org/10.1016/j.scitotenv.2018.12.076
[8] R. Bond, S. Veerapaneni, Zero Liquid Discharge for Inland Desalination, Black and Veatch, 2007.
[9] W. Shih et al., Chino II Desalter concentrate management via innovative by-product resale and treatment, American Membrane Technology Association, San Antonio, TX, 2013.
[10] L .F. Greenlee et al., Effect of antiscalant degradation on salt precipitation and solid/liquid separation of RO concentrate, J. Membr. Sci. 2011, 366, 48–61. https://doi.org/10.1016/j.memsci.2010.09.040
[11] F. Alimi et al., Kinetics of the precipitation of calcium sulfate dihydrate in a desalination unit, Desalination 2003, 158, 9–16. https://doi.org/10.1016/S0011-9164(03)00426-0
[12] R. Sheikholeslami, H. W. K. Ong, Kinetics and thermodynamics of calcium carbonate and calcium sulfate at salinities up to 1.5 M, Desalination 2003, 157, 217–234. https://doi.org/10.1016/S0011-9164(03)00401-6
[13] J. E. Goldman et al., Selective Salt Recovery from Reverse Osmosis Concentrate. Report Foundation, W.R.-61, 2013.
[14] Major Industrial Applications uses of CaCO3 calcium carbonate in everyday life, usmasterbatch.com. (accessed on March 2, 2022)
[15] Uses Of Magnesium Hydroxide, www.osianmcpl.com. (accessed on March 2, 2022)
[16] P. Silvestroni (Eds.: M. Pasquali, A. Latini), Fondamenti di Chimica, CEA, 2020. ISBN: 9788808920539
[17] C. Akilan et al., Temperature effects on ion association and hydration in MgSO4 by dielectric spectroscopy, ChemPhysChem 2006, 7, 2319–2330. https://doi.org/10.1002/cphc.200600342
[18] R. Molinari et al., Selective precipitation of calcium ion from seawater desalination reverse osmosis brine, J. Cleaner Production 2021, 329, 129645. https://doi.org/10.1016/j.jclepro.2021.129645
[19] T. Chen et al., Influence of Mg2+ on CaCO3 formation-bulk precipitation and surface deposition, Chem. Eng. Sci. 2006, 61, 5318–5327. https://doi.org/10.1016/j.ces.2006.04.007
[20] Y. Zhang, R. A. Dawe, Influence of Mg2+ on the kinetics of calcite precipitation and calcite crystal morphology, Chem. Geol. 2000, 163, 129–138. https://doi.org/10.1016/S0009-2541(99)00097-2
[21] Y. Choi et al., Effect of chemical and physical factors on the crystallization of calcium sulphate in seawater reverse osmosis brine, Desalination 2018, 426, 78–87. https://doi.org/10.1016/j.desal.2017.10.037
[22] K. S. Pitzer, The Treatment of Ionic Solutions over the Entire Miscibility Range, Ber. Bunsengesellschaft Phys. Chemie. 1981, 85, 952–959. https://doi.org/10.1002/bbpc.19810851107
[23] D. L. Parkhurst, C. A. J. Appelo, Description of Input and Examples for PHREEQC Version 3 – A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport and Inverse Geochemical Calculations, U.S. Geological Survey Techniques and Methods 2013, 6, A43, 497.
[24] M. Telzhensky et al., Selective separation of seawater Mg2+ ions for use in downstream water treatment processes, Chem. Eng. J. 2011, 175, 136–143. https://doi.org/10.1016/j.cej.2011.09.082
[25] M. Reig et al., Integration of nanofiltration and bipolar electrodialysis for valorization of seawater desalination brines: Production of drinking and waste water treatment chemicals, Desalination 2016, 382, 13–20. https://doi.org/10.1016/j.desal.2015.12.013
[26] Y. Wang et al., Selective removal of calcium ions from seawater or desalination brine using a modified sodium carbonate method, Desal. Water Treat. 2020, 174, 123–135. https://doi.org/10.5004/dwt.2020.24828
Authors
Raffaele Molinari*1, Ahmet Halil Avci1, Pietro Argurio1, Efrem Curcio1, Sandra Meca2, Sandra Casas2, Mireia Plà-Castellana2, Hannah Arpke2, Jose Luis Cortina3
1 Department of Environmental Engineering (DIAm), University of Calabria, via P. Bucci, cubo 44/A, Rende (CS), Italy.
2 Sustainability Area, EURECAT, Centre Tecnològic de Catalunya, Spain
3 Chemical Engineering Department, Universidad Politécnica de Cataluña · Barcelona Tech (UPC), Spain
* Corresponding author





I am just an inquisitive ordinary person. After reading this and a few other articles on this topic I still have concerns.
My questions are:
1. After all the separation and extraction from the brine what remains?
A huge concentration of salt?
What happens to this? Thrown in the sea/ocean as final disposition? Doesn’t that greatly increases the salt/saline content in the water? What that will
mean for the marine environment, marine life?
2. Also, heavier salt/salinity in sea/ocean will not adversely affect the earth’s atmosphere? Like causing, among others, salinity in the clouds and rains?
3. Is there any danger of increased salinity in sea/ocean water seeping into not only land near to the rivers and canals as well as in acquifer/acquirers? What will be the consequences of that?
There are many other reasons for concerns/grave concerns. But for now I will be waiting to hear from you at least on these questions.
Regards.