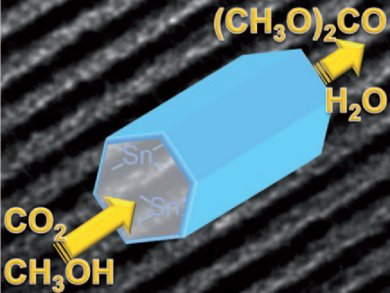
The conversion of CO2 to dialkyl carbonates and polycarbonates requires the use of harmful feedstocks such as phosgene and CO and suffers from low conversions. The direct route to dimethyl carbonate (DMC) via the oxidative carbonylation and carbonation of methanol has the potential to avoid these harmful feedstocks, achieve 83 wt % atom efficiency, and produce only water as a by-product.
Danielle Ballivet-Tkatchenko and co-workers, University of Bourgogne, France, have developed a mesoporous material that selectively catalyzes the coupling of CO2 with methanol to give DMC. The material contains organotin species within its pore channels. The mesopores are believed to stabilize the tin species and prevent leaching. Because of this, the material gave the highest activity reported so far in the absence of water traps and had good recyclability.
Image: © Wiley-VCH
- Tin-Based Mesoporous Silica for the Conversion of CO2 into Dimethyl Carbonate
D. Ballivet-Tkatchenko, F. Bernard, F. Demoisson, L. Plasseraud, S. R. Sanapureddy,
ChemSusChem 2011.
DOI: 10.1002/cssc.201100034