Nitrile functionalities are one of the key structural motifs in organic and industrial chemistry. They allow entry to the synthesis of pharmaceuticals, agrochemicals, and polymers.
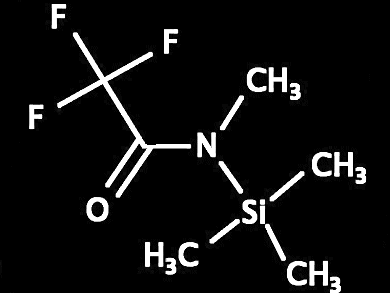
Stephan Enthaler, Technische Universität Berlin, Germany, investigated the straightforward iron-catalyzed dehydration of a variety of aromatic and alkyl amides to the corresponding nitriles by using N-methyl-N-(tri-methylsilyl)trifluoroacetamide (MSTFA) as the dehydration reagent. After optimization of the reaction conditions, excellent catalytic activities and selectivities were feasible.
- Straightforward Iron-Catalyzed Synthesis of Nitriles by Dehydration of Primary Amides,
Stephan Enthaler,
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201100754


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
