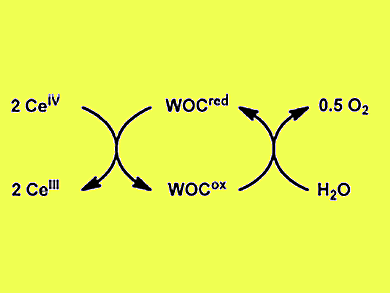
Photocatalytic water splitting is an attractive choice for the production of hydrogen in a clean and sustainable manner. Studies thus far have mainly considered the water-splitting process as two independent half-reactions, of which water oxidation is the more challenging one. In the water oxidation half-reaction a sacrificial oxidant is used to drive the catalytic cycle (pictured).
Matthias Beller and his team, Leibniz Institute for Catalysis, Rostock, Germany, show that simple, commercially available iridium precursors such as iridium(III) chloride or iridium(III) acetyl acetonate are highly active for water oxidation. The obtained turnover ferquencies (TOFs) are as high as 1700 h–1 (for IrCl3), and can compete with the majority of much more complicated organometallic iridium complexes.
- Simple and Efficient Iridium(III)-Catalyzed Water Oxidations,
Nicolas Marquet, Felix Gärtner, Sebastian Losse, Marga-Martina Pohl, Henrik Junge, Matthias Beller,
ChemSusChem 2011.
DOI: 10.1002/cssc.201100217