The inclusion of small hydrocarbons into molecular container compounds — such as pumpkin-shaped cucurbit[n]uril (CBn) macrocycles — in solution complements solid-state applications and has potential applications in gas storage, uptake, and separation. CB6 (above) is the ideal size to include n-butane in its cavity, but has lower water solubility than other cucurbiturils, which makes the determination of binding constants difficult.
Mara Florea and Werner Nau, Jacobs University Bremen, Germany, have used a fluorescent dye displacement approach to determine gas binding constants of CB6 in salt-free aqueous solutions (see scheme). Volatile hydrocarbons were shown to bind very tightly and selectively. As proof of CB6’s selectivity, an unidentified impurity was removed from an aqueous solution of neopentane.
The obtained set of binding constants is the most extensive one for the binding of guest mlecules with cucurbiturils, and will act as a benchmark for future studies.
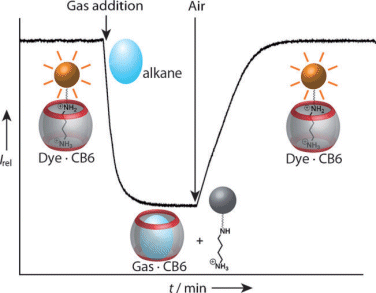
Image: © Wiley-VCH
- Strong Binding of Hydrocarbons to Cucurbituril Probed by Fluorescent Dye Displacement: A Supramolecular Gas-Sensing Ensemble
M. Florea, W. M. Nau,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201104119