Catalyst systems that are highly efficient, robust, easily obtained, environmentally benign, rely only on earth-abundant elements, and work in aqueous solution without needing any organic solvent are desired for the production of H2 on large scales.
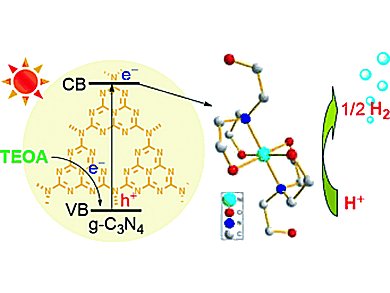
Mei Wang, Licheng Sun, and colleagues, Dalian University of Technology (DUT), China, combined a molecular catalyst system containing only earth-abundant elements with graphitic carbon nitride (g-C3N4) as photosensitizer. g-C3N4 has attracted much attention because of its good thermal and chemical stability, small band gap (2.67 eV), corresponding to an absorption up to 460 nm, as well as its convenient preparation from cheap organic monomers.
Iin situ generated complexes [M(TEOA)2]Cl2 (M = Ni, Co; TEOA = triethanolamine) serve as catalysts for visible-light-driven H2 production in aqueous solution and do not require organic solvent or a Brønsted acid.
The nickel-based system displays the highest catalytic activity in TEOA aqueous solution, and the H2 evolution lasts more than 60 h. This is considerably longer than the lifetime of H2 production of previously reported systems involving earth-abundant metal complexes as catalysts in aqueous solution.
The results prove that the combination of effective organic polymer light-harvesting materials with non-precious-metal complexes is a promising approach to improve the catalytic lifetimes of catalyst systems in photochemical H2 production.
- Simple Nickel-Based Catalyst Systems Combined With Graphitic Carbon Nitride for Stable Photocatalytic Hydrogen Production in Water,
Jingfeng Dong, Mei Wang, Xueqiang Li, Lin Chen, Yu He, Licheng Sun,
ChemSusChem 2012.
DOI: 10.1002/cssc.201200490