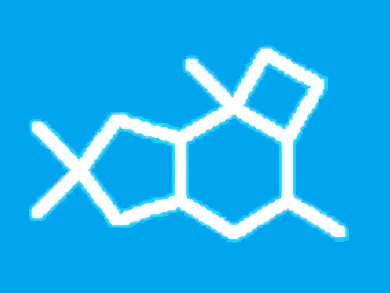
Sesquiterpenes containing the characteristic 5/6/4-protoilludane framework have fascinating biological properties and members of this natural product class are of interest as lead structures for the development of new antibiotics. Protoilludanes also offer great potential for synthetic endeavors.
Peter Siengalewicz, Johann Mulzer and Uwe Rinner, University of Vienna, Austria, took the fortieth anniversary of the first protoilludane synthesis as an occasion to review the main aspects of this class of compounds. Biosynthesis, biological properties and the unusually rich variety of synthetic approaches developed over the past fourty years are discussed.
The first synthesis of racemic illudol, and at the same time the first synthesis of a naturally occurring protoilludane, was reported by T. Matsumoto in 1971. The key steps in this seminal contribution are a [2+2] cycloaddition to establish the cyclobutane moiety and a subsequent ring expansion reaction to construct the cyclohexane ring.
A multitude of approaches for the construction of the tricyclic framework, above all the annulation of the cyclobutane ring, have
been developed since then. Straightforward [2+2] photocycloaddition has been a standard option throughout, although Diels–Alder or ketene olefin cycloaddition, cyclopropyl ring enlargement or free radical or cobalt mediated enediyne [2+2+2] cyclizations have emerged as surprisingly useful alternatives.
- Synthesis of Protoilludanes and Related Sesquiterpenes,
Peter Siengalewicz, Johann Mulzer, Uwe Rinner,
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201101220