Chemistry can achieve miracles in many applications, but miracles have no place in chemistry as a natural science. However, chemistry occasionally surprises us with puzzles that seem unsolvable and can at least verge on the miraculous. One such riddle is Emil Fischer’s capricious hydrazone. We return to the year 1896 and look over his shoulder as he determines the melting point.
 |
|
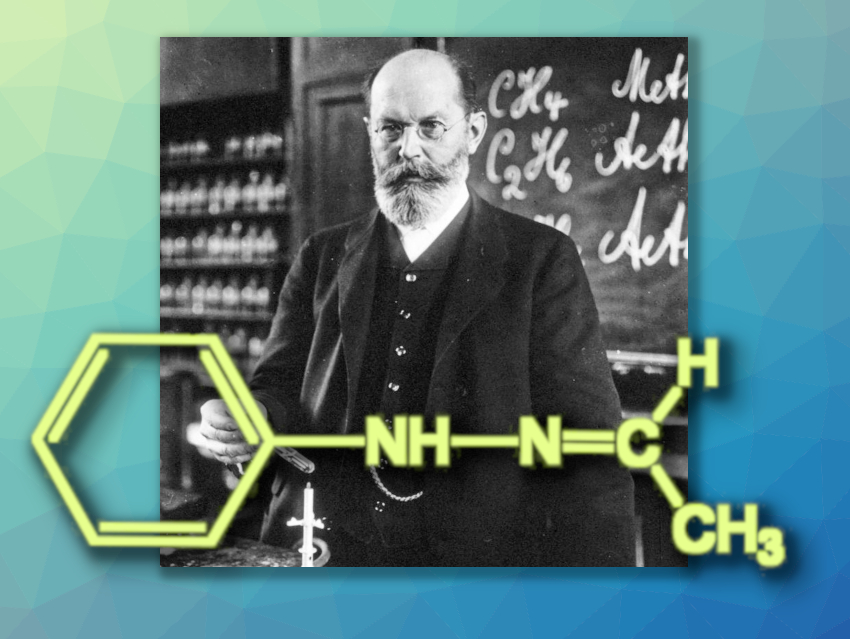
Figure 1. Emil Fischer in 1912, performing a recrystallization. |
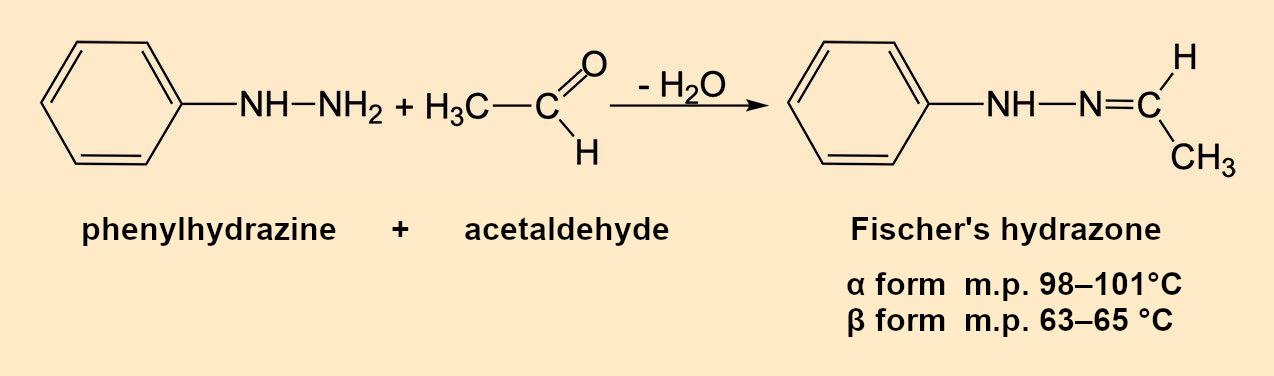
Emil Fischer (1852–1919) obtained a high yield of colorless crystals that melted at 80 °C when he reacted “his” phenylhydrazine (see Infobox 1) [1,2] with acetaldehyde (ethanal). After distillation, recrystallization, and drying, the melting point (m.p.) was 63–65 °C (without decomposition). Elemental analysis and molecular mass corresponded to the molecular formula C8H10N2 and were in agreement with Fischer’s expectation (see Fig. 2): Acetaldehyde phenyl hydrazone, or 1-ethylidene-2-phenylhydrazine, according to IUPAC.
 |
|
Figure 2. Synthesis of Fischer’s hydrazone. |
1. The Antics of Fischer’s Hydrazone
 Emil Fischer produced his hydrazone a number of times and wondered about its highly variable melting point. After recrystallization of the crude product from petroleum ether, the melting point of 65 °C rose unexpectedly to 80 °C, sometimes even higher; after longer drying in the desiccator, it dropped to 60 °C.
Emil Fischer produced his hydrazone a number of times and wondered about its highly variable melting point. After recrystallization of the crude product from petroleum ether, the melting point of 65 °C rose unexpectedly to 80 °C, sometimes even higher; after longer drying in the desiccator, it dropped to 60 °C.
The only explanation Fischer had for this was the existence of a second, higher-melting crystalline form. (Fischer uses the terms isomeric “compounds” or “forms”. Because the structural relationship between the α and β forms of his hydrazone remained unclear until recently, we will use Fischer’s terminology in describing the investigations carried out before 1911.) In his search for this form, he found a simple method to obtain the higher-melting α form. The lower-melting β crystals (m.p. 60 °C) were simply dissolved in ethanol, reacted with a little 40 % sodium hydroxide, and cooled. The resulting precipitate consisted of pure α crystals (m.p. 98–101 °C, without decomposition). Both crystalline forms “differ in solubility and outward crystallization habit” [3]. The molecular mass and elemental analysis confirm a molecular formula of C8H10N2 [3,4] for both.
Despite precise work, the melting points varied between 60 °C and 100 °C, and Fischer could never be sure if further purification would cause the melting point to rise, fall, or remain constant. Even storage in the desiccator changed the melting point. As the leading representative of preparative organic chemistry around 1900, and a legendary experimenter, such inexplicable fluctuations in one of the most important physical properties of a compound he had prepared did not suit him in the least. Fischer expressly referred to this lack of reproducibility—a nightmare of every experimental natural scientist—in his publication:
“Under conditions that I was not able to determine, there was sometimes a sudden lowering of the melting point, which drops to 80 °C or even lower. There is probably a conversion back to the β compound.”
1.1. “Mr. Causse” Makes Trouble
The fluctuating melting points of Fischer’s hydrazone would have quickly been forgotten as a curiosity if the French chemist H. Causse from Nancy had not also produced the hydrazone and published a report [5–7]. Based on the elemental analysis, H. Causse derived a molecular formula of C18H22N4 for the reaction product. This corresponded to a 3:2 condensation product of acetaldehyde and phenylhydrazine (triéthylenidène diphénylhydrazine). He realized that there was a relationship between the melting point of the reaction product and the acid or base content of the reaction medium. Like Fischer, he ascribed this to the existence of two crystalline forms with melting points of 60 °C and 99.5 °C.
Fischer was likely “not amused” when he read about Causse’s work, because an interloper in one’s own field of research is rarely welcome. In his reply, published in 1897, Fischer “ignored” that Causse could not know of his work of 1896, and began his riposte smugly:
“Mr. Causse has found that in aqueous solution, depending on whether it is alkali, neutral, or acidic, products of different melting points result. He examined two of these more precisely. Both supposedly have the formula C18H22N4. The first melts at 60 °C and the second at 99.5 °C. These are nearly the same melting points that I found for the two isomers of hydrazone. M. Causse did not carry out molecular weight determinations, although these would best have been able to differentiate between the two very different formulas, C8H10N2 and C18H22N4.”
At the end of the publication, Fischer dealt his knock-out blow, in which his harsh verdict was additionally highlighted by the spacing of the print:
“B a s e d o n t h e a f o r e m e n t i o n e d r e s u l t s , I d o n o t h e s i t a t e t o d e c l a r e a l l a n a l y t i c a l r e s u l t s o f M r . C a u s s e r e g a r d i n g t h e p r e v i o u s l y n o t e d p r o d u c t s a n d a l l s p e c u l a t i o n s b a s e d o n t h e s e r e s u l t s t o b e f a l s e.”
Who would want to get on the wrong side of the great Emil Fischer? He was the leading organic chemist and had, in his negotiations upon his appointment to the Friedrich Wilhelms University in Berlin, Germany, negotiated the building of a new institute, an adjacent villa with a private laboratory and two private assistants. In academic circles at the time, it was said that “if Helmholtz recommended someone, they were as good as offered the position, if Fischer recommended someone, they were as good as hired.” No wonder that “Mr. Causse” meekly adopted Fischer’s interpretation after this attack.
From today’s point of view, Fischer’s withering verdict was overblown, because Causse did discover the influence of acids and bases on the resulting crystalline form. Triéthylenidène diphénylhydrazine was indeed formed in the reaction and melted at 109–110 °C [8]. An X-ray crystallographic analysis of this compound has been carried out [9].
1.2. Fischer Obtains Support from Zurich
This passionate frenzy made other research groups aware of the melting-point muddle. Eugen Bamberger and W. Pemsel at the Swiss Federal Institute of Technology in Zurich, Switzerland, simplified the synthesis of Fischer’s hydrazone by simply adding cooled acetaldehyde dropwise to a solution of phenylhydrazine in ether (see Fig. 2) [10].
The yield of snow-white crystals was 95–96 %. The melting point varied between 90–96 °C and rose to 96–97 °C upon recrystallization from petroleum ether. Further vacuum distillation did not change the melting point. This was inconsistent with Fischer’s result, as he obtained pure β crystals after distillation (m.p. 60 °C).
Bamberger and Pemsel elegantly avoided a confrontation with Fischer by representing this contradiction as further proof of the “peculiar relationships” and “uncontrollable influences” mentioned by Fischer:
“We have briefly debated these peculiar relationships to confirm Fischer’s observation that uncontrollable influences play a prole in the rearrangement of both acetaldehyde hydrazones.”
1.3. Fischer Obtains Support from Leipzig
Georg Lockemann and Otto Liesche at the University of Leipzig, Germany, picked up the topic again in 1905, agreeing with Fischer in their publication [11]:
“Causse’s work about the phenylhydrazine compounds of acetaldehyde does not even need to be taken into account, since it can be viewed as not only unreliable, but completely backward, as shown by E. Fischer’s results and his critique; a verdict that Causse tacitly deferred to in a later paper.”
The Leipzig group also had their hands full with Fischer’s hydrazone:
“The melting point of the freshly prepared hydrazone was usually low, between 51 °C and 57 °C. In individual cases, higher melting points of up to 98 °C were also observed, effects of the often-mentioned ‘uncontrollable influences’.”
However, the Leipzig team did not let up, wanting to get to the bottom of Fischer’s “uncontrollable influences”. The first step was to develop more robust synthetic procedures for both crystalline forms. Whereas Fischer’s procedure for the α form could be reproduced without a hitch, Fischer’s synthesis of the β form proved to be highly unreliable. The Leipzig team was in an unenviable position:
“The method specified by Fischer failed and usually depended on happenstance, until a precise observation of all circumstances that could possibly come into consideration finally pointed in the right direction.”
This “right direction” became clear thanks to their sharp powers of observation: The Leipzig team stored the freshly synthesized α crystals in a desiccator under hydrogen gas to prevent any oxidation. This showed that:
“The melting point of the α-hydrazone sank after storage in the desiccator if it was filled with hydrogen. However, this effect was not attributed to the hydrogen itself, but to a tiny trace of sulfurous acid—which forms when the gas is washed with concentrated sulfuric acid—that entered the desiccator. Turning off the sulfur dioxide vapors allowed the high-melting hydrazone to be stored unchanged.”
The Leipzig researchers immediately put their newly won knowledge to work. Fischer’s hydrazone, whether as a crude or purified product, regardless of melting point, was recrystallized from 75 % alcohol to which some sulfurous acid had been added. This caused pure β crystals to precipitate. This rearrangement also worked with hydrochloric acid, nitric acid, or dilute sulfuric acid.
With both crystalline forms in hand, Lockemann and Liesche were able to closely study the transformation of one to the other. They determined that recrystallization in the presence of tiny amounts of base yielded preferentially the α form, and that the presence of acid led to the β crystals. This transformation even occurred in the solid state. (According to E. G. Laws and N. V. Sidgwick [12], the mixed melting points change constantly and in correspondence with the composition.)
When exposed to gaseous ammonia or sulfur dioxide, the corresponding α or β crystals are formed. Clearly, the two crystalline forms easily convert from one to the other, so that the melting point after purification by recrystallization could never be predicted. The unusual melting behavior of the two crystalline forms could not be explained with the measurement methods available at the time. They gave up, and Fischer’s unsolved case did eventually fade from memory.
How the case is taken up again in 2008, causing further confusion before it becomes clear that everyone was somehow right, will be reported in the second part of this chemistry puzzle.
References
[1] E. Fischer, Ueber aromatische Hydrazinverbindungen, Ber. Dtsch. Chem. Ges. 1875, 8, 589–594. https://doi.org/10.1002/cber.187500801178
[2] E. Fischer, Ueber die Hydrazinverbindungen; Erste Abhandlung, Liebigs Ann. 1878, 190, 67–183. https://doi.org/10.1002/jlac.18781900104
[3] E. Fischer, Ueber die Phenylhydrazone der Aldehyde, Ber. Dtsch. Chem. Ges. 1897, 30, 1240–1243. https://doi.org/10.1002/cber.18970300216
[4] E. Fischer, Ueber das Azophenyläthyl und das Acetaldehydphenylhydrazon, Ber. Dtsch. Chem. Ges. 1896, 29, 793–797. https://doi.org/10.1002/cber.189602901143
[5] H. Causse, Sur les aldéhydates de phénylhydrazine, Bull. Soc. Chim. Paris 1896, 15, 842.
[6] H. Causse, Sur l’action de l’éthanyl sur la phénylhydrazine et sur deux triéthylidène-diphénylhydrazines isomèriques α et β, Bull. Soc. Chim. Paris 1897, 17, 234.
[7] H. Causse, Sur les aldéhydates de phénylhydrazine, Comptes Rendus 1896, 122, 1274.
[8] H. Causse, Action de l’aldéhyde èthylique sur la phénylhydrazine, Bull. Soc. Chim. Paris 1898, 18, 145.
[9] C. Bernades et al., The Curious Case of Acetaldehyde Phenylhydrazone: Resolution of a 120 Year Old Puzzle where Forms with Vastly Different Melting Points Have the Same Structure, Cryst. Growth Des. 2019, 19, 2, 907–917. https://doi.org/10.1021/acs.cgd.8b01459
[10] E. Bamberger, W. Pemsel, Zur Kenntniss des Acetaldehydphenylhydrazons, Ber. Dtsch. Chem. Ges. 1903, 36, 85–89. https://doi.org/10.1002/cber.19030360115
[11] G. Lockemann, O. Liesche, Zur Kenntniss des Aethylidenphenylhydrazins, Liebigs Ann. 1905, 342, 14–50. https://doi.org/10.1002/jlac.19053420103
[12] E. G. Laws, N. V. Sidgwick, Isomeric acetaldehydephenylhydrazones, J. Chem. Soc. Trans. 1911, 99, 2085–2093. https://doi.org/10.1039/CT9119902085
The article has been published in German as:
- Emil Fischers ungelöster Fall,
Klaus Roth,
Chem. unserer Zeit 2020, 54, 378–385.
https://doi.org/10.1002/ciuz.202000039
and was translated by Caroll Pohl-Ferry.
Emil Fischer’s Unsolved Case: Cleared Up after 120 Years – Part 2
The unpredictable properties of both crystalline forms of Fischer’s hydrazone
See similar articles by Klaus Roth published on ChemistryViews