The importance of nitrogen-containing heterocycles cannot be underestimated in the fields of natural product synthesis and pharmaceuticals. Benito Alcaide, Universidad Complutense de Madrid, Spain, and co-workers have reported a synthesis of such biologically active heterocycles through a simple and effective reaction. They examined the controlled ring expansion of isatin derivatives mediated by trimethylsilyldiazomethane leading to insertion of a methylene group. This reaction was catalyzed by scandium triflate to afford quinolin-2-one derivatives (see scheme).
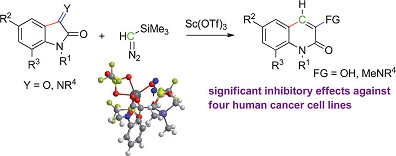
The study includes density functional calculations that underline the proposed mechanism for the ring enlargement and biological testing, which shows that several synthesized compounds have potent cytotoxic activity against several cell lines. The most potent compound gave IC50 values of approximately 0.7±0.1.
- Scandium-Catalyzed Preparation of Cytotoxic 3-Functionalized Quinolin-2-ones: Regioselective Ring Enlargement of Isatins or Imino Isatins,
B. Alcaide, P. Almendros, C. Aragoncillo, G. Gómez-Campillos, M. Arnó, L. R. Domingo,
ChemPlusChem 2012.
DOI: 10.1002/cplu.201200090
This article is available for free as part of the ChemPlusChem free trial.