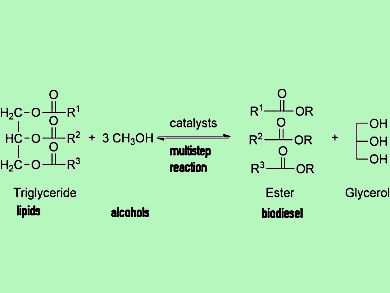
Biodiesel (fatty acid methyl esters; FAMEs) can be produced from lipids from plants, animal, or oleaginous yeast through a transesterification reaction between the lipids (triglycerides) and alcohols (e.g., methanol) catalyzed by basic or acidic catalysts. This is a multistep process involving reactions, extractions, and separations (pictured).
The catalytic systems must be neutralized, which gives rise to wastewaters, they cannot be reused, favor the formation of stable emulsions making separation of the FAMEs difficult, and are sensitive to the presence of water and free of fatty acids. Glycerol is obtained as an aqueous solution of relatively low purity.
Switchable solvents are based on an exothermic transformation from an organic base, an alcohol, and an acid gas (e.g. CO2). They are capable of reversible compositional change under mild conditions from molecular liquids to ionic liquids, in association with other switching properties, such as polarity and viscosity. This introduces a variety of possible applications in catalysis and separation processes.
Bo Jing, Xiangtan University, China, and colleagues found that by using switchable solvents like 1,8-diazabicyclo-[5.4.0]undec-7-ene (DBU) with methanol, CO2 can be used as a trigger to catalyze the methanolysis of soybean and microbial lipids.
The team found that the FAMEs can be decanted from the system in a 95.2 % conversion efficiency. The produced glycerol can be extracted from the FAMEs completely by the “switchable solvents”, and can be separated with high purity after recycling the DBU by an easy extraction process.
The study presents an efficient and facile process for the integrated clean production of biodiesel and glycerol from soybean and microbial lipids, and the catalyst DBU can be recycled by an easy extraction process.
- Phase-switching Homogeneous Catalysis for Clean Production of Biodiesel and Glycerol from Soybean and Microbial Lipids,
Xuefeng Cao, Haibo Xie, Zhilian Wu, Hongwei Shen, Bo Jing,
ChemCatChem 2012.
DOI: 10.1002/cctc.201200150