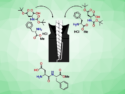
Grinding nitroolefins, β-ketoesters, and a cinchona-alkaloid organocatalyst together with a pestle and mortar is a high-yielding and highly stereoselective method for conducting Michael additions, as demonstrated by Pankaj Chauhan and Swapandeep Singh Chimni, Guru Nanak Dev University, India,
The experimental protocol is simple: The olefin, ester, and catalyst are ground together for five minutes then left to stand until the reaction is complete. The desired Michael adducts, which contain vicinal quaternary and tertiary stereocenters, were obtained in yields of up to 99 % with up to 98 % enantiomeric excess and up to 99:1 diastereomeric ratio.
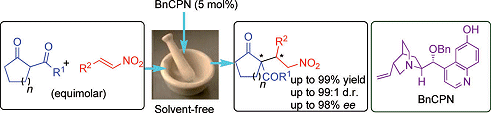
These grinding-assisted reactions meet some of the most important criteria for environmentally benign chemistry, as there are no solvents and no metal-based catalysts involved. The team is currently investigating the scope of this mechanochemistry for other syntheses.
 Grinding-Assisted Asymmetric Organocatalysis: A Solvent-free Approach to the Formation of Vicinal Quaternary and Tertiary Stereocenters,
Grinding-Assisted Asymmetric Organocatalysis: A Solvent-free Approach to the Formation of Vicinal Quaternary and Tertiary Stereocenters,
Pankaj Chauhan, Swapandeep Singh Chimni,
Asian J. Org. Chem. 2012.
DOI: 10.1002/ajoc.201200048