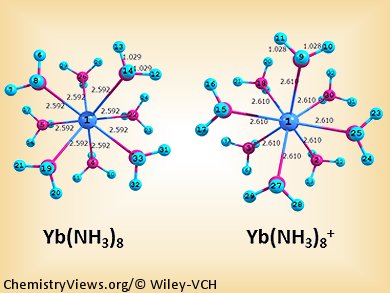
Ytterbium dissolves in liquid ammonia in a fashion similar to alkali metals. This was shown for the first time by experimental measurements by Andrew M. Ellis and co-workers, University of Leicester, UK. Their data is complimented by state-of-the-art calculations that were used to assign the structure of the [Yb(NH3)n] complexes formed, and reproduced the experimental results obtained.
They found that the ytterbium is embedded within a shell of ammonia molecules, rather than sitting on the exterior of a hydrogen-bonded (NH3)n cluster, as is found for [Mg(NH3)n] complexes. The calculations also suggest that Yb can accommodate up to eight NH3 molecules in its first solvation shell before steric repulsion makes occupancy of the second solvation shell more favourable energetically. Using a laser they measured the energy required to remove an electron from the clusters. The ionization energies of the studied ytterbium complexes closely follow those measured for complexes of alkali metal atoms with NH3 molecules, suggesting that a valence electron detaches from the Yb atom to form a solvated electron in Yb(NH3)n when n is sufficiently large.
These results provide insight into the relative strength of solute–solvent and solvent–solvent interactions and improve our understanding of how metal atoms interact with solvents.
- Photoionization of Yb(NH3)n Complexes,
Matthew J. Guttridge, Sadna H. Don, Andrew M. Ellis,
ChemPhysChem 2012.
DOI: 10.1002/cphc.201200691