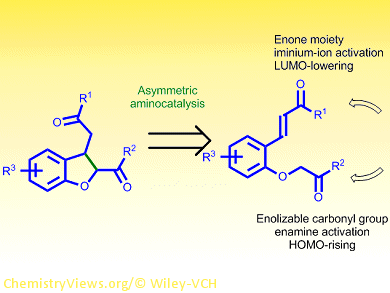
General, efficient, and enantioselective routes to cis-2,3-dihydrobenzofurans remain scarce. Karl Anker Jørgenson and co-workers, Aarhus University, Denmark, have developed an organocatalytic procedure to produce these biologically relevant compounds with high yield and selectivity.
The researchers used the open-chain precursor (E)-3-(2-(2-oxopropoxy)phenyl)-1-phenylprop-2-en-1-one to study the intramolecular Michael addition to form the corresponding dihydrobenzofuran. A primary aminocatalyst derived from a chinchona alkaloid proved effective in the presence of an acid additive. At room temperature, the cyclic product was obtained quantitatively with 95 % enantiomeric excess and 2.5:1 diastereomeric ratio in favor of the cis diastereomer.
Many different substrates could be used in this reaction, and compounds bearing both electron-rich and electron-poor groups as well as those with ortho-, meta-, and para– substitution at the aromatic moiety gave good results. Experiments at different temperatures suggested that the cis diastereomer was kinetically favored, while the trans product was thermodynamically more stable. This conclusion was supported by 1H NMR spectroscopy over an extended period of time.
- Stereocontrolled Organocatalytic Strategy for the Synthesis of Optically Active 2,3-Disubstituted cis-2,3-Dihydrobenzofurans,
Jannie Christensen, Łukasz Albrecht, Karl Anker Jørgensen,
Chem. Asian J. 2012.
DOI: 10.1002/asia.201201072