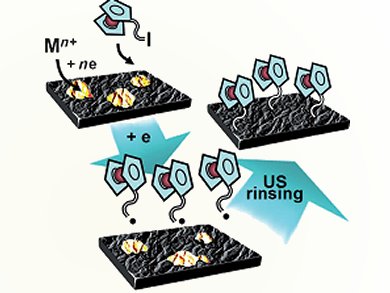
Surface immobilization of functional groups, sought for in many modern applications, is traditionally realized by the reduction of diazonium salts. French researchers Viatcheslav Jouikov and Jacques Simonet have developed a new paradigm of radical grafting to carbon-based interfaces allowing covalent binding of functional groups through alkyl linkers.
Their process permits attaching redox markers and various functional groups using simple alkylbromides as starting material. The key of the process is an in situ exchange of Br for I, promoted by polar solvents containing tetrabutylammonium iodide, and trapping the resulting alkyl iodides by oxidative addition on the preliminary surface-deposited catalytic amounts of transition metals (Pd, Pd but also Cu, Ni, Co etc). One-electron reduction of these metallorganic surface-located transients produces alkyl radicals efficiently attacking Csp2 sites of the graphitic interface and regenerates the catalyst.
Owing to very moderate cathodic potentials required for this process and its high efficiency, it is suitable for direct grafting of various functional groups to glassy carbon, carbon fibers, industrial coke, and other graphitic materials. A multistep postfunctionalization, unavoidably following the diazonium grafting, is no longer necessary in the present method: the substrate to be grafted only needs to possess a bromoalkyl branch, such derivatives are often commercially available or easy to synthesize.
- Grafting of ω-Alkyl Ferrocene Radicals to Carbon Surfaces By Means Of Electrocatalysis with Subnanomolar Transition-Metal (Pd, Pt, or Au) Layers,
Viatcheslav Jouikov, Jacques Simonet,
ChemPlusChem 2013.
DOI: 10.1002/cplu.201200261