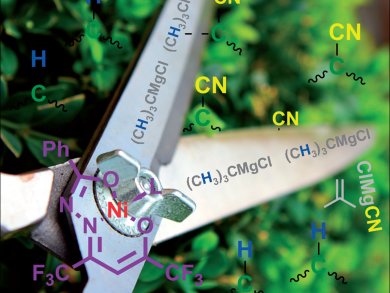
Cyanide groups are useful in organic synthesis for directing reactions to occur at specific positions on phenyl rings. These cyanide groups often need to be removed after performing the directing function and Stephan Enthaler and co-workers, Technische Universität in Berlin, Germany, have now demonstrated a nickel-based system that can help do just that.
The technique works by mixing the substrate and a nickel-based precatalyst with the Grignard reagent tert-butylmagnesium chloride. The researchers suggest that the Grignard reagent and the nickel precatalyst first combine to form a nickel-magnesium hydride species. A radical mechanism is then initiated, which results in the replacement of the aromatic cyanide group with a hydrogen atom. The team is now examining the mechanism of this hydrodecyanation reaction in more detail.
- Nickel-catalyzed Hydrodecyanation of Carbon–Cyano Bonds,
Maik Weidauer, Chika I. Someya, Elisabeth Irran, Stephan Enthaler,
Asian J. Org. Chem. 2013, 2(2), 150–156.
DOI: 10.1002/ajoc.201200185


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
