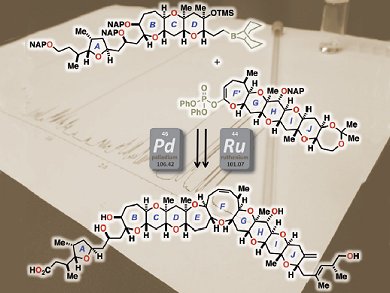
Gambieric acids (GAs) A–D are potent antifungal marine polycyclic ethers, which were isolated from Gambierdiscus toxicus. Their skeletal structure is a nonacyclic polyether core containing 6-, 7-, and 9-membered cyclic ethers, arranged with an isolated tetrahydrofuran ring (A ring). GAs share structural characteristics with polycyclic ether neurotoxins produced by G. toxicus, such as ciguatoxin, maitotoxin, and gambierol, and exhibit potent antifungal activity against Aspergillus niger.
Although the structure and relative stereochemistry of the polycyclic ether domain of GAs have been determined previously, recent studies on the synthesis of A/B-ring model compounds suggest that the relative stereochemical relationship between the C9 and C11 stereogenic centers was incorrectly assigned.
.gif)
Haruhiko Fuwa, Makoto Sasaki, and co-workers, Tohoku University, Japan, have now unambiguously established that the altered structure is correct by achieving the first total synthesis of (+)-gambieric acid A. The synthesis relied on a Suzuki–Miyaura reaction to assemble the A/BCD- and F′GHIJ-ring fragments and ring-closing metathesis for the formation of the nonacyclic backbone. Moreover, they evaluated the antifungal and antiproliferative activities of (+)-gambieric acid A and several analogues to investigate their structure–activity relationships.
- Total Synthesis and Biological Evaluation of (+)-Gambieric Acid A and Its Analogues,
Kazuya Ishigai, Haruhiko Fuwa, Keisuke Hashizume, Ryo Fukazawa, Yuko Cho, Mari Yotsu-Yamashita, Makoto Sasaki,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201204303