Audio Cassettes Make the Production Process for Fuels Less Expensive
To produce nanoparticles made of inexpensive iron oxide cores with a very thin cobalt shell, an international team of researchers modified a method developed for the production of magnetic audio tapes. As the researchers report in the journal Angewandte Chemie, their particles are easily accessible on a large scale, and are excellent Fischer–Tropsch catalysts for the production of good diesel fractions.
The increasing importance of shale gas and natural gas is bringing a century-old process back into the limelight: The Fischer–Tropsch synthesis, an industrial process for the liquefaction of coal developed in 1925, involves the catalytic conversion of a carbon monoxide/hydrogen mixture (synthesis gas) into gaseous and liquid hydrocarbons. These days, it is used in some countries for the production of ultrapure synthetic fuels from coal or natural gas. Biomass is also a good feedstock for this process.
Choice of Cobalt or Iron
The success of this process depends entirely on the catalyst, whose active component can be iron or cobalt. Each of these metals has advantages and disadvantages and one is chosen over the other based on the properties of the gas feed. Most large firms use cobalt, the major disadvantage of which is its price.
But, since only the surface of the catalytic particles is involved, one alternative is using particles with a core made of a less expensive material covered with a thin layer of the expensive, active material. However, this requires both nanometric accuracy and a cost-effective, simple, and scalable process for producing the catalytic particles, to ensure that they will still be cheaper than pure cobalt.
Core–Shell Catalysts using Modified Cassette Production Process
A Dutch, French, and German team led by Gadi Rothenberg at the University of Amsterdam, the Netherlands, together with Total Gaz & Energies Nouvelles, Paris, France, has now met this challenge by inventing new core–shell catalysts, inspired by patents from the 1960s for producing audio cassettes. The magnetic tapes used in these cassettes were coated with cigar-shaped iron oxide particles covered with a thin cobalt layer. By modifying this process, the researchers succeeded in making the spherical particles needed for catalysis.
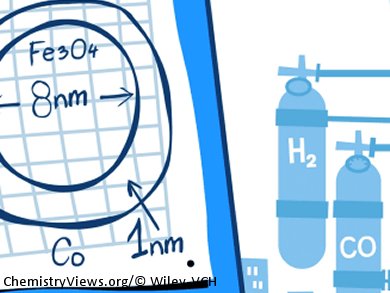
The production process involves the synthesis of iron oxide nanoparticles from an iron chloride solution. Addition of a cobalt nitrate solution causes a thin layer of cobalt oxide to coat the nanoparticles. This allows the researchers to produce particles with a diameter of about 10 nm, consisting of an 8 nm iron oxide core and a whisper-thin, 1 nm cobalt-rich shell. These particles were processed with an alumina support to make pellets. To activate the catalyst, the pellets were heated under a hydrogen atmosphere, selectively reducing the cobalt oxide to metallic cobalt.
Tests in Fischer–Tropsch reactors at Lille and Bayreuth shows that the resulting particles are effective and robust catalysts. The product composition shows that the iron is also participating in the catalysis. There is clearly an iron–cobalt cooperative effect that has not been investigated before.
- De Novo Design of Nanostructured Iron–Cobalt Fischer–Tropsch Catalysts,
V. Roberto Calderone, N. Raveendran Shiju, Daniel Curulla-Ferré, Stéphane Chambrey, Andrei Khodakov, Amadeus Rose, Johannes Thiessen, Andreas Jess, Gadi Rothenberg,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201209799
On the occasion of the 125th anniversary of Angewandte Chemie, a one-day symposium is held on March 12 with several Nobel laureates. Learn more and join the free webcast or recording at chemistryviews.org/angewandtechemie125.