The conversion of dinitrogen to ammonia or higher value organonitrogen compounds by using homogeneous transition metal complexes has been a topic of long-standing interest in inorganic chemistry.
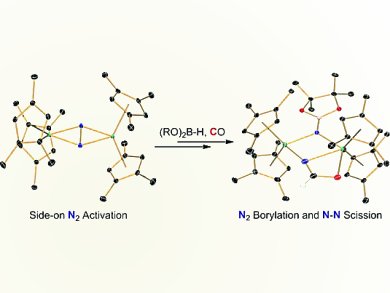
Scott P. Semproni and Paul J. Chirik, Princeton University, NJ, USA, successfully demonstrated that zirconocene and hafnocene complexes with side-on bound dinitrogen ligands participate in a wide variety of nitrogen–element bond forming reactions, including hydrogenation, carboxylation, CO-induced N2 cleavage, and silylation. The robust nature of the cyclopentadienyl rings prevents unwanted participation of ancillary ligation and allows construction of more elaborate nitrogen-based ligands following N2 functionalization.
They now report a new highly activated zirconocene dinitrogen compound supported by 1,2,4-trimethylcyclopentadienyl ligands. This compound, along with its hafnium congener, [(η5-C5H2-1,2,4-Me3)2Hf] 2(μ2,η2,η2-N2), promoted clean N2 borylation, the first examples of B–N bond formation in group 4 metallocene dinitrogen chemistry. Subsequent addition of cyclohexanecarbonitrile resulted in insertion into the bridging hydride ligand while carbonylation resulted in N–N bond scission with N–C bond formation. In contrast, the reaction of tertbutylisocyanide with the boryldiazenidohafnocene complex resulted in exclusive hydride insertion with no evidence for N–N bond cleavage.
- Dinitrogen Borylation with Group 4 Metallocene Complexes,
Scott P. Semproni, Paul J. Chirik,
Eur. J. Inorg. Chem. 2013.
DOI: 10.1002/ejic.201300046