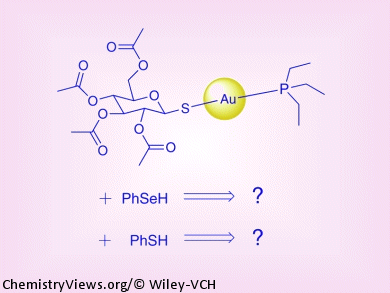
The thioredoxin/thioredoxin reductase (Trx/TrxR) enzyme system, which is present in all living cells, is responsible for cell proliferation in mammalian organisms. Its catalytic site contains a redox-active selenocysteine (Sec) residue. The affinity of selenium to electrophilic heavy metals makes the system sensitive to inhibition by electrophilic complexes of heavy metals, for example by auranofin, a gold-containing compound that has shown promise as an anticancer drug.
Alessandro Bagno and co-workers, Università di Padova, Italy, report a study of the reactions of auranofin with models of thiol and selenol nucleophiles (PhSH and PhSeH) that are present in TrxR. The reactions were investigated under a variety of experimental conditions by 1H, 31P, and 77Se NMR spectroscopy, and the elementary processes involved were characterized by DFT calculations. Complex patterns of reactivity are revealed, such as auranofin undergoing ligand substitution of the tetraacetylthioglucose moiety by a PhS or PhSe group in chloroform. The reaction is reversible in both cases, but it is characterized by widely different equilibrium constants.
The information provided by this combination of methods about the protonation/substitution reactions with auranofin has notable implications for the development of new anticancer drugs.
- Reactivity of Auranofin with Selenols and Thiols – Implications for the Anticancer Activity of Gold(I) Compounds,
Francesca Di Sarra, Barbara Fresch, Riccardo Bini, Giacomo Saielli, Alessandro Bagno,
Eur. J. Inorg. Chem. 2013.
DOI: 10.1002/ejic.201300058