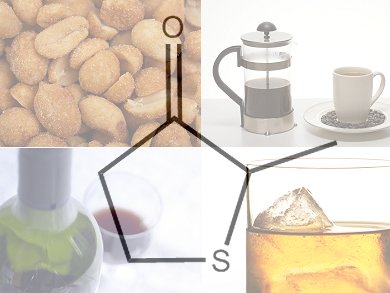
Smelly Sulfur – From Sweet to Life-Saving
Volatile sulfur compounds are among the smelliest chemicals we know. The stench of a skunk is due to such compounds, grapefruit and garlic have sulfurous components and, of course, hydrogen sulfide is the smell of rotten eggs and various bodily processes. However, at very low concentrations H2S simply smells sweet, and immediately before the rise to toxic concentration it has no smell.
A tiny quantity of another sulfur compound, mercaptan, is commonly added to natural gas because, ironically, the sulfur contaminants present when it is piped from the ground are removed before it’s piped to consumers. The mercaptan makes the otherwise odorless methane “smell of gas” and has saved many lives.
Sulfur compounds can be noxious and toxic but low concentrations present in or added to food and drink usually enhance the overall flavor. Three very disparate consumables—coffee, roasted peanuts, and wine and whiskey—contain the sulfur compound dihydro-2-methyl-3-thiophenone. This compound is also known as blackberry thiophenone and adds a characteristic fruitiness to aromas depending on the other congeners present in the food or drink. Sulfur compounds are often used when different meat, garlic, onion, or other vegetable flavors are needed in a food product.
Dihydro-2-methyl-3-thiophenone
Halima Mouhib, Vinh Van, and Wolfgang Stahl of the Institute of Physical Chemistry, RWTH Aachen University, Germany, have used quantum chemical calculations and molecular beam Fourier transform microwave spectroscopy to investigate dihydro-2-methyl-3-thiophenone in detail in the gas phase; the most interesting phase for the olfactory system, you might say.[1]
The focus is on gaining a better understanding of the structure of sulfur compounds, many of which are present in foods. Dihydro-2-methyl-3-thiophenone is a superficially simple molecule that is a component of the aroma/flavor of many different foods, as are its derivatives.
The team carried out the spectroscopy at 3.0 to 26.5 GHz on 97 % pure dihydro-2-methyl-3-thiophenone. They then used the Xiam software to extract three rotational constants and five quartic centrifugal distortion constants at high accuracy for two different conformers.
They reveal details of its conformers and what the researchers describe as “highly accurate rotational constants and centrifugal distortion constants,” supported by theoretical predictions at the MP2/6-311++G(d,p) level. Such structural details are important for studies into how derivatives might behave in terms of aroma and other factors.
Oxygen versus Sulfur
The team points out that understanding the physical chemistry of such sulfur compounds is important for the food and drink industry. Understanding what it is about such a molecule that gives it its characteristic odor will offer food chemists and those devising simple, inexpensive flavorants the opportunity to modify their products.
Intriguingly, the team points out that derivatives of smelly sulfur compounds, in which the sulfur atom has been replaced by an oxygen atom, are usually not at all malodorous. Dihydro-2-methyl-3-thiophenone itself is not strongly malodorous, unlike the thiols, and its oxygen analog, 2-methyldihydrofuran-3(2H)-one, smells of bread.
Compared with many of the strongly smelling sulfur compounds, one might think that dihydro-2-methyl-3-thiophenone is of little interest, although it too is repellent to the human nose at higher concentrations. However, the biosynthetic route to the compound, as it is made by yeasts and bacteria, was revealed in the bacterium Chitinophaga Fx7914 and this additional knowledge about the compound has piqued the interest of flavor chemists and organic chemists alike.[2]
Particularly intriguing, are the possibilities that structural and conformer studies of this and other smelly sulfur compounds raise in terms of the synthesis of derivatives with slightly or even markedly different aromas for use in the food and drink industry. Conventional chemical syntheses might be complemented with the use of engineered microbes to biosynthesize analogs of this particular compound for further testing. Moreover, the physiological activity of such compounds might pique the interest of the pharmaceutical or agrochemical industries looking for novel drug and pest deterrents from near-natural sources.
[1] Sulfur-Containing Flavors: Gas Phase Structures of Dihydro-2-methyl-3-thiophenone,
Halima Mouhib, Vinh Van, Wolfgang Stahl,
J. Phys. Chem. A 2013.
DOI: 10.1021/jp4041748
[2] The Biosynthesis of the Aroma Volatile 2-Methyltetrahydrothiophen-3-one in the Bacterium Chitinophaga Fx7914,
Thorben Nawrath, Klaus Gerth, Rolf Müller, Stefan Schulz,
ChemBioChem, 2010, 11, 1914–1919.
DOI: 10.1002/cbic.201000296