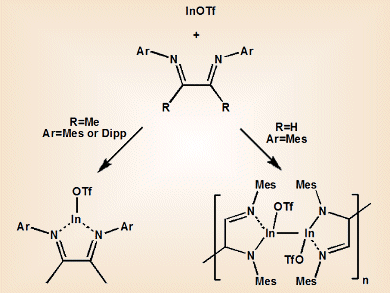
When coordinated to a metal, “non-innocent” ligands can undergo redox reactions that make simple electron counting impossible and assignment of the metal’s oxidation state difficult. 1,4-diazabutadienes (DAB) are of interest for forming group 13 equivalents of N-heterocyclic carbenes (NHCs). However, attempts to coordinate neutral ligands to low oxidation state indium centers are often hindered by “non-innocent” behavior. This leads to disproportionation pathways that produce elemental indium and higher oxidation state species.
Charles L. B. Macdonald and colleagues, University of Windsor, Canada, have shown that In-based equivalents of NHCs can be synthesized by using relatively stable indium(I) triflate, InOTf, instead of indium halides. The team explored the effect of substituents on the DAB ligand on the resultant complex. They found that InOTf•MesDABMe (MesDABMe=N,N-dimesityl-2,3-dimethyl-diazabutadiene) formed a discrete molecular compound with a pyramidal coordination at the indium center. This structure is consistent with the presence of a lone pair of electrons on indium and a neutral diazabutadiene chelate ligand. This “innocent” behavior of the DAB ligand is unprecedented for indium(I) and lighter monovalent group 13 species.
In contrast, use of the less electron-rich MesDABH ligand (MesDABH=N,N-dimesityl-diazabutadiene) gave dramatically different reactivity, producing a metallopolymer linked by C–C and In–In bonds.
- Non-Innocent Ligand Effects on Low Oxidation State Indium Complexes,
Christopher J. Allan, Benjamin F. T. Cooper, Hugh J. Cowley, Jeremy M. Rawson, and Charles L. B. Macdonald,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201301881