Tert-alkylamine groups, in particular 1-azaspirocycles, are found in many pharmaceuticals and bioactive natural products, including alkaloids. These groups can be synthesized from amides, which are highly stable and readily available. However, this transformation normally requires several steps. The development of a direct transformation of tertiary lactams/amides into tert-alkylamines would, thus, open up access to many bioactive natural products.
Pei-Quang Huang and co-workers, Xiamen University, China, have reported a method for the bis-alkylation of amides and lactams to directly give tert-alkylamines. The strategy involves activating the corresponding lactam or amide with triflic anhydride to form reactive intermediate (1). The transformation is reminiscent of the use of POCl3 in the Vilsmeier reaction. Subsequent treatment with organometallic reagents results in the addition of two alkyl groups to (1), to give the final product. Importantly, the stepwise addition of two organometallic reagents allows two distinct alkyl groups to be introduced (see scheme below).
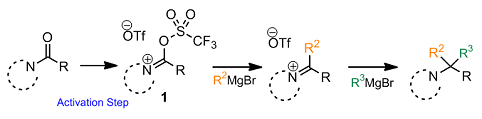
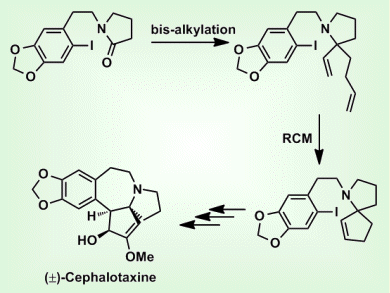
The procedure was used to produce a series of 1-azaspirocycles through bis-alkylation followed by ring closing metathesis (RCM). This combined bis-alkylation/RCM strategy was also applied to the synthesis of (±)-cephalotaxine (pictured above).
- General One-Pot Reductive gem-Bis-alkylation of Tertiary Lactams/Amides: Rapid Construction of 1-Azaspirocycles and Formal Total Synthesis of (±)-Cephalotaxine,
Kai-Jiong Xiao, Jie-Min Luo, Xiao-Er Xia, Yu Wang, Pei-Qiang Huang,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201302096