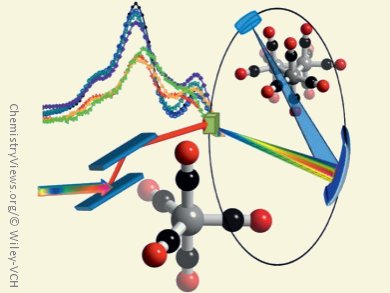
X-ray absorption spectroscopy (XAS) is often used to understand metal-catalyzed reactions, for example, the EXAFS (Extended X-Ray Absorption Fine Structure) region can provide details about metal–ligand bond distances. However, EXAFS cannot distinguish between elements that have similar scatter patterns, such as C, N, and O. Differentiating between a carbonyl and an alkyne is not feasible by EXAFS. In transition-metal-catalyzed reactions of CO, transition metal/carbonyl complexes are key intermediates. However, as other ligands, such as hydrocarbons, may also bind to the metal center it is difficult to study such reactions by EXAFS.
Mario Delgado-Jaime, Serena DeBeer, both Max-Planck-Institut für Chemische Energiekonversion, Mülheim, Germany, and Matthias Bauer, Technische Universität Kaiserslautern, Germany, have investigated valence-to-core X-ray emission spectroscopy (V2C XES) as a probe for understanding the transformation of CO. The team applied V2C XES to series of compounds relevant to both homogeneous catalysts and intermediates in heterogeneous reactions, namely [Fe(CO)5], [Fe2(CO)9], [Fe3(CO)12], [Fe(CO)3(cod)] (cod = cyclo-octadienyl), [Fe2Cp2(CO)4] (Cp = yclo-pentadienyl), [Fe2Cp*2(CO)4] (Cp* = tetramethylcyclopentadienyl), and [FeCp(CO)2(thf)][B(ArF)4] (ArF = pentafluorophenyl).
They found that the resultant spectra can be deconvoluted into corresponding ligand and metal fragment contributions and that both metal 3p and 4p orbitals may contribute to the observed valence-to-core intensity. The observed experimental intensity depends on the nature of the ligand and metal molecular orbitals. This explains the greater sensitivity of V2C XES to CO σ-bonding interactions, while demonstrating why there is essentially no contribution from the CO π orbitals.
This observation allows for the separation of carbonyl and hydrocarbon signals. Additionally, the potential for time-resolved measurements means that V2C XES can be used to follow the changes in ligation at metal centers during the course of chemical reactions.
- Valence-to-Core X-Ray Emission Spectroscopy of Iron–Carbonyl Complexes: Implications for the Examination of Catalytic Intermediates,
Mario Ulises Delgado-Jaime, Serena DeBeer, Matthias Bauer,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201301913