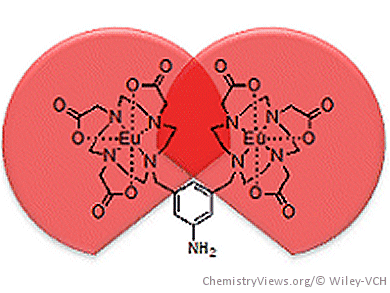
Lanthanide ions are widely used in optical applications and magnetic-resonance imaging (MRT). In particular, the long-lived emission from f–f transitions from lanthanide ions such as Eu3+ and Tb3+ can be exploited in luminescence imaging. Use of lanthanides in vivo requires kinetically stable lanthanide complexes to avoid complications arising from the presence of free lanthanide, as lanthanide release has been implicated in the onset of nephrogenic systemic fibrosis.
Stephen Faulkner, Oxford University, UK, and colleagues have studied the anion-binding properties of three structurally related lanthanide complexes (1–3; see Figure below).
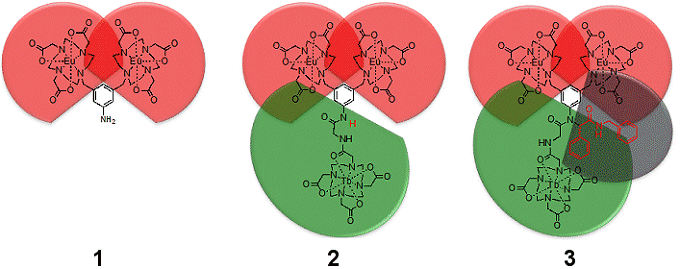
The team created the complexes with modified versions of the hepta-dentate ligand DO3A (1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid). It has bulky substituents added at positions away from the binding pocket. These resulted in dramatic changes to the binding constant for isophthalate despite an identical binuclear binding pocket in each complex.
The difference in association constant, which ranged over two orders of magnitude from 1·105 M–1 for 1, through 2·106 M–1 for 2, to 1·107 M–1 for 3, was attributed to the remote substituents restricting the available conformational space. Thus, in the peptide-coupled system 2, the terbium domain impinged upon the conformational freedom of the europium domains and dictated the structure of the binding pocket to some degree.
This restriction increased the affinity of the complex for isophthalate and gave robust hepta-dentate binding pockets that ensured that the lanthanide remained complexed throughout the experiment.
- Using Remote Substituents to Control Solution Structure and Anion Binding in Lanthanide Complexes,
Manuel Tropiano, Octavia A. Blackburn, James A. Tilney, Leila R. Hill, Matteo P. Placidi, Rebecca J. Aarons, Daniel Sykes, Michael W. Jones, Alan M. Kenwright, John S. Snaith, Thomas Just Sørensen, Stephen Faulkner,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201303183