Systems showing photoswitching of electronic communication between redox-active moieties could be used as part of intelligent molecule-based electronic circuits controlled by light sources. The ability to connect redox-active molecules and linker molecules that have very close redox potentials is one approach to making redox-active molecules with the required strong electronic communication and photoswitching properties.
Dimethyldihydropyrene (DHP) is an organic photochromic molecule with reversible redox properties. As the redox potential of its one-electron oxidation is very close to that of ferrocene (Fc), Hiroshi Nishihara, University of Tokyo, Japan, and colleagues designed two bis(ferrocenyl)DHP complexes. Bis(ferrocenylethynyl)benzodimethyldihydropyrene (1), in which two Fc moieties are connected to benzodimethyldihydropyrene (BzDHP) by an ethynyl linkage, shows reversible photoswitching of the electronic communication between the Fc moieties in THF, but not in dichloromethane. In (2) the Fc groups were replaced by pentamethylferrocenyl groups. It showed no photoswitching behavior, even in THF.
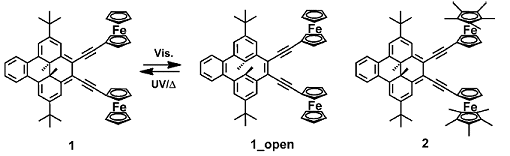
Analysis of the redox potentials showed that the mixed-valence state of (1) was more stable in dichloromethane than in THF and that of (2) was much less stable than that of (1). The stronger electronic communication in (1) was due to the small energy gap between the Fc moieties and the BzDHP moiety in (1) compared with that in (2). This finding was supported by DFT calculations.
The team concludes that connecting redox centers with photochromic linkers that have a redox potential close to that of the redox centers is a useful approach for the production of photochromic redox-active metal complexes with strong electronic communication.
- Comparative Study of Photochromic Ferrocene-conjugated Dimethyldihydropyrene Derivatives,
Satoshi Muratsugu, Masa-aki Kishida, Ryota Sakamoto, Hiroshi Nishihara,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201303456