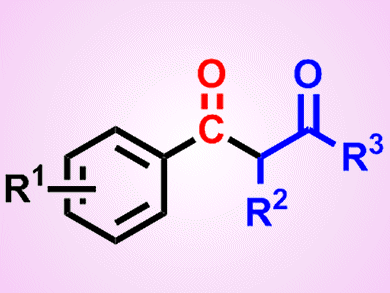
Palladium-catalyzed carbonylative coupling between an aryl halide or a derivatized phenol and a carbon- or heteroatom-based nucleophile represents a powerful method for the generation of a diverse array of benzoic acid and aryl ketone derivatives. It has thus generated great interest in the scientific community. Nevertheless, a particular but important drawback when working with CO is its inherent toxicity, resulting in asphyxiation upon its inhalation due to its competition with oxygen for binding to hemoglobin.
Thomas Gøgsig, Anders Lindhardt, Troels Skrydstrup, and co-workers, Aarhus University, Denmark, have identified reaction conditions for the three-component synthesis of aryl 1,3-diketones. The synthesis involves a palladium-catalyzed carbonylative α-arylation of ketones with aryl bromides in the presence of a catalytic system generated from [Pd(dba)2] and the bidentate ligand DPPP (1,3-bis(diphenylphosphino)propane).
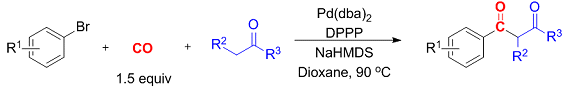
Importantly, these reactions are run in the presence of only stoichiometric amounts of carbon monoxide generated from COgen (9-methylfluorene-9-carbonyl chloride) in the two-chamber reactor, COware. The reactions are adaptable to access a diverse range of aryl 1,3-diketones.
31P and 13C NMR spectroscopic analyses of a series of reactions with stoichiometric amounts of palladium complexes were also undertaken to provide mechanistic information on the catalytic cycle of the reactions.
- Pd-Catalyzed Carbonylative α-Arylation of Aryl Bromides: Scope and Mechanistic Studies,
Dennis U. Nielsen, Camille Lescot, Thomas M. Gøgsig, Anders T. Lindhardt, Troels Skrydstrup,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201303384