Photo-assisted heterogeneous reactions on TiO2 particles have attracted much attention because of their promising applications in solar energy conversion, water splitting, and treatment of environmental contamination. However, insight into, and deep comprehension of, the induced chemical reactions is lacking. For example, in the degradation of organic carboxylic acids in water, the final step by which the organic compounds release CO2, still remains unclear.
Wanhong Ma, Jincai Zhao, and co-workers, Beijing National Laboratory for Molecular Sciences, Chinese Academy of Sciences, have studied the degradation behaviors of five straight-chain dicarboxylic acids, from ethanedioic acid to hexanedioic acid, in aqueous TiO2-based photocatalysis.
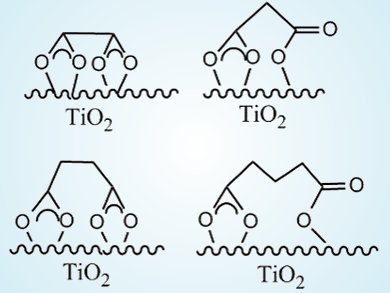
When all other conditions were identical, dicarboxylic acids with an even number of carbon atoms (e-DAs) always degraded more slowly than those acids with an odd number of carbon atoms (o-DAs). By using attenuated total reflection FTIR of 13C-labeled dicarboxylic acids, the team showed that both carboxyl groups of e-DAs coordinate to the TiO2 surface through bidentate chelating forms (see picture, left). In contrast, only one carboxyl group of the o-DAs coordinated to TiO2 in a bidentate chelating manner, the other forming a monodentate binding linkage (right).
Further studies showed nearly all of the oxygen in the pentanedioic acid degradation product (butanedioic acid) came from H2O, whereas only half of the oxygen in the butanedioic acid degradation product (propanedioic acid) came from H2O. This result was explained by the asymmetrical chelation of o-DAs, which permitted all oxidant species, even dioxygen, to approach and attack the weakened C1–C2 bond associated with the monodentate chelation. On the other hand, the strong symmetrical bidentate chelation of e-DAs effectively restrained any approach or attack by an active species. Thus, this unusual fluctuation in the reactivity is significantly affected by the coordination patterns of the substrates on the TiO2 surface.
- An Unexpected Fluctuating Reactivity for Odd and Even Carbon Numbers in the TiO2-based Photocatalytic Decarboxylation of C2-C6 Dicarboxylic Acids,
Yiran Sun, Wei Chang, Hongwei Ji, Chuncheng Chen, Wanhong Ma, Jincai Zhao,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201303236