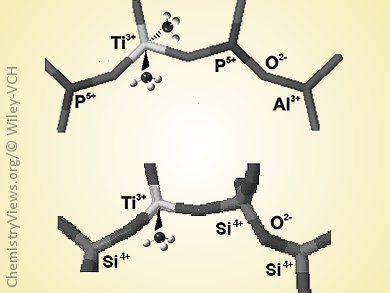
The incorporation of transition-metal ions in the framework of micro- and mesoporous materials generates isolated sites that often lack structural precedent in molecular chemistry. A prototypical example is the insertion of titanium ions in the frame of silicalite to generate titanium silicalite-1 (TS-1), one of the most extensively used catalysts for selective oxidation of hydrocarbons for the functionalization of petroleum-based feedstocks. The Ti4+ sites in TS-1 have been extensively studied, but much less is known about the structure of the reduced Ti3+ species generated in the framework.
Mario Chiesa and co-workers, University of Torino, Italy, have followed the reaction of triethylaluminum with TS-1 by continuous-wave and pulse electron paramagnetic resonance (EPR) and HYSCORE spectroscopies that detect 29Si hyperfine interactions. The results provide evidence for the reducing power of triethylaluminum towards isolated Ti4+ ions through the formation of open-shell Ti3+ ions. The Ti3+ ions in TS-1 were shown to preferentially have five-fold coordination, at variance with the tetrahedral Ti3+ ions in the framework of porous aluminophosphate molecular sieves.
This result provides a simple and effective route to generate accessible tetrahedrally coordinated open-shell Ti3+ sites in silica frames, which may open new reaction pathways in coordination chemistry and redox catalysis. This approach could also be extended to other oxide or halide systems to better understand the surface chemistry of alkyl aluminum compounds and activation-deactivation mechanisms in heterogeneous Ziegler–Natta systems.
- Probing the Redox Chemistry of Titanium Silicalite-1: Formation of Tetrahedral Ti3+ Centers by Reaction with Triethylaluminum,
Elena Morra, Elio Giamello, Mario Chiesa,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304139