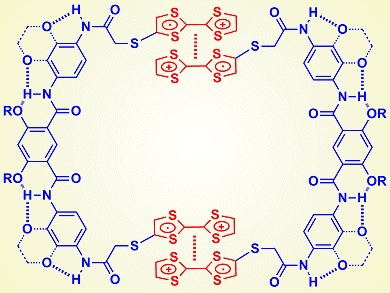
Tetrathiafulvalene (TTF) is an important electron-rich unit in supramolecular and materials chemistry. The donor–acceptor interaction between TTF and viologen, bipyridinium derivatives of 4,4′-bipyridyl, plays a central role in supramolecular chemistry. Although it is well-known that TTF undergoes reversible reduction and oxidation, for many years the stacking of its radical cation has been studied by fixing the molecule into an interlocked system or cavity. The reason is simple: the stacking is weak.
Zhan-Ting Li and colleagues, Fudan University, Shanghai, China, and Yi Liu, The Molecular Foundry, Berkeley, USA, reported that, upon attaching two TTF units to a hydrogen-bonding-induced foldamer linker, the stacking or dimerization of the corresponding TTF radical cations can be enhanced remarkably to drive the molecule to form a uni- or bimolecular macrocycle. The apparent equilibrium constant of this process is as high as 104 M–1 in dichloromethane.
The researchers comment that the result is important because aromatic stacking is usually weak in common organic solvents such as dichloromethane and chloroform. Their result suggests that the strong dimerization of the TTF radical cation may be developed as a new binding motif for supramolecular self-assembly in organic solvents.
- Tetrathiafulvalene-Based Macrocycles Formed by Radical Cation Dimerization: The Role of Intramolecular Hydrogen Bonding and Solvent,
Wei-Kun Wang, Yuan-Yuan Chen, Hui Wang, Dan-Wei Zhang, Yi Liu, Zhan-Ting Li,
Chem. Asian. J. 2014.
DOI: 10.1002/asia.201301729