The development of new luminescent materials based on π-conjugated organic molecules is important because of their applications in electronics, optics, storage media, and biological sciences. Such compounds are usually used in the aggregated state for practical applications. However, a common problem associated with the emission of most luminescent materials is aggregation-caused quenching (ACQ): although fluorescent materials are highly emissive in the solution state, they become weakly emissive or even non-emissive when aggregated in the condensed phase. This effect has significantly limited the high-tech application of conventional fluorophores.
In 2001, Ben Zhong Tang, Hong Kong University of Science & Technology, China, and co-workers observed the phenomenon of aggregation-induced emission (AIE) [1]. It is the exact opposite of ACQ.
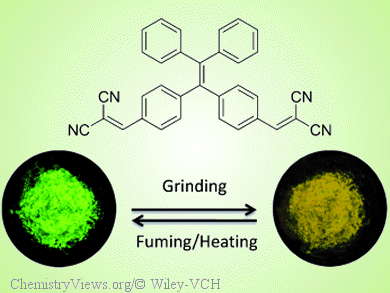
Tang and colleagues now report a yellow AIE luminogen (TPE-DCV), which was synthesized by treating tetraphenylethene with two dicyanovinyl units. The solid-state emission of TPE-DCV can be reversibly switched between green and yellow by grinding then fuming with acetone vapor or heating because of a morphological change between the thermodynamically stable crystalline phase and the metastable amorphous state.
This demonstrates that TPE-DCV can serve as a recyclable optical storage medium. In addition, crystalline microrods and microslices of TPE-DCV are excellent optical waveguides with very low optical loss.
- An Aggregation-Induced Emission Luminogen with Efficient Luminescent Mechanochromism and Optical Waveguiding Properties,
Na Zhao, Chuang Zhang, Jacky W. Y. Lam, Yong Sheng Zhao, Ben Zhong Tang,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300223
[1] J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu, B. Z. Tang, Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun. 2001, 1740–1741. DOI: 10.1039/B105159H