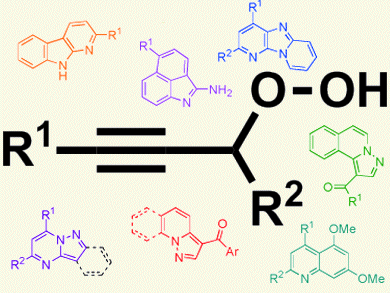
Organic hydroperoxides can be found in many biologically active natural products; however, despite the potential of these compounds and several approaches reported for their synthesis, their synthetic utility has been somewhat limited.
Following their recent report of the preparation of propargylic hydroperoxides and their subsequent gold-catalyzed rearrangements to ketones and concomitant alcoholic trapping [1], Benito Alcaide and co-workers, Universidad Complutense de Madrid, Spain, report the gold-catalyzed reactions of propargylic hydroperoxides with a variety of aromatic amines. These reactions are perfomed in the presence of catalytic amounts of a protic acid. They involve an interesting tandem sequence to produce azaheterocycles, a diverse family of biologically active molecules.
There are no previous examples with amine nucleophiles, which could prove valuable for the construction of structurally new heterocyclic systems. Therefore, the ability to employ aromatic amines in the rearrangement of propargylic hydroperoxides provides an attractive alternative to an otherwise stepwise azaheterocycle synthesis and a platform to further expand the synthetic utility of the propargylic hydroperoxide rearrangement.
- Gold/Acid-Co-catalyzed Direct Microwave-Assisted Synthesis of Fused Azaheterocycles from Propargylic Hydroperoxides,
Benito Alcaide, Pedro Almendros, M. Teresa Quirós,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304509
[1] B. Alcaide, P. Almendros, M. T. Quirós, R. López, M. I. Menéndez, A. Sochacka-Ćwikła, J. Am. Chem. Soc. 2013, 135, 898–905. DOI: 10.1021/ja3108966