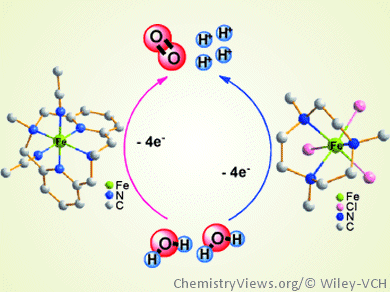
In artificial photosynthesis, the water oxidation reaction is often the sticking point. The reaction of two water molecules to one dioxygen molecule, four electrons, and four protons is uphill energetically.
A group of researchers led by Licheng Sun, Royal Institute of Technology, Stockholm, Sweden, and Dalian University of Technology, China, sought an inexpensive, readily available homogeneous catalyst for this reaction and discovered a novel catalytically active iron complex with a macrocyclic four-nitrogen-donor ligand. The team screened a total of twenty different complexes with different chelating nitrogen- and oxygen-donor ligands.
The best performance was found for the complex [Fe(L-N4Me2)(CH3CN)2](OTf)2 (L-N4Me2 = N,N’-dimethyl-2, 11-diaza[3.3](2,6)pyridinophane), which showed a turnover number of 65 and an initial turnover frequency of 0.1 s–1. This complex proved to be among the most active earth-abundant metal catalysts for water oxidation. The researchers attribute its high activity to the excellent stability of macrocyclic iron complexes under acidic conditions.
- Homogeneous Oxidation of Water by Iron Complexes with Macrocyclic Ligands,
Biaobiao Zhang, Fei Li, Fengshou Yu, Honghua Cui, Xu Zhou, Hua Li, Yong Wang, Licheng Sun,
Chem. Asian J. 2014.
DOI: 10.1002/asia.201400066