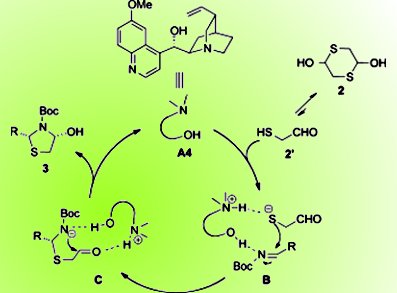
Thiazolidines and their analogues are important structural motifs in biologically active molecules and are versatile intermediates in organic synthesis. The previous asymmetric syntheses of chiral thiazolidines mostly start from chiral pool materials, which can be more expensive than the less-studied catalytic asymmetric strategies from achiral materials.
A solution to this problem is now at hand courtesy of Jianwei Sun and co-workers, Hong Kong University of Science and Technology, Hong Kong SAR, China. With readily available imines and 2-mercaptoacetaldehyde as the starting materials, a formal [3+2] annulation is efficiently catalyzed by quinidine to enantio- and diastereoselectively assemble various substituted thiazolidines. The reaction starts with activation of both reaction partners by the bifunctional catalyst. Then the sulfur addition to the imine is stereoselectively controlled by the chiral backbone. Subsequent cyclization by addition of the nitrogen atom to the aldehyde moiety forms the desired product.
The reaction tolerates different aryl imines, and the team hopes to extend the method to other types of imines and/or other catalytic systems to improve stereoselectivity.
- Organocatalytic Enantio- and Diastereoselective Assembly of Thiazolidine Scaffolds by Formal [3+2] Annulation,
Hui Qian, Jianwei Sun,
Asian J. Org. Chem. 2014, 3, 387–390.
DOI: 10.1002/ajoc.201400025