Molecular catalysis of electrochemical reactions has received renewed interest, because of the issues raised by new energy challenges. Reductive and oxidative transformations of small molecules, such as H2O, O2, and CO2, have severe activation problems, which require catalysis. Many molecular catalysts have been proposed, but their catalytic activity must be compared. In catalyst benchmarking, the onset potentials can provide a rough estimate of the maximal (reduction) and minimal (oxidation) electrode potential that is required in order for catalysis to be possible.
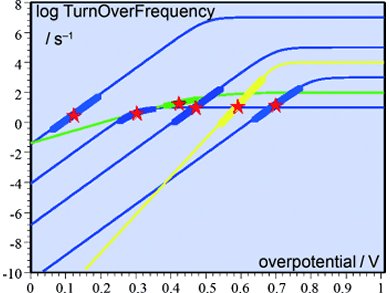
Jean-Michel Savéant and Cyrille Costentin, Université Paris Diderot, France, use a more rigorous approach to benchmark catalysts. It consists of plotting the turnover frequency (TOF) as a function of the overpotential (η) for each catalyst. The equations and procedures are generalized to obtain catalytic Tafel plots for multielectron, multistep molecular catalytic processes. The researchers focus on two-electron, two-step systems in which the catalyst is present in the solution rather than it being deposited onto the electrode surface.
Based on their results, the η at which electrolysis is conducted and the desired TOF can be optimized, and a compromise between these two parameters can be identified. The way in which benchmarking can be conducted within a TOF versus η plane of merit is illustrated by thought examples, showing logTOF versus η plots of various reaction schemes with arbitrary, but typical, parameters of different catalysts of the same reaction.
This analysis can be used to identify the optimal molecular catalyst system for various electrochemical reactions.
- Multielectron, Multistep Molecular Catalysis of Electrochemical Reactions: Benchmarking of Homogeneous Catalysts,
Cyrille Costentin, Jean-Michel Savéant,
ChemElectroChem 2014, 7.
DOI: 10.1002/celc.201300263