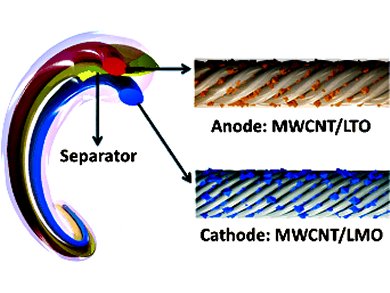
Wire-shaped Lithium-ion Battery
Flexible smartphones, “intelligent” bracelets, glasses with a built-in computer: for these trends to take off, we need suitable power systems. Chinese scientists have developed a wire-shaped lithium ion battery that contains electrodes consisting of two composite yarns made of carbon nanotubes and lithium titanium oxide or lithium manganese oxide. As the researchers report in the journal Angewandte Chemie, they were able to weave their batteries into light, flexible, elastic, and safe textile batteries with a high energy density.
Previous methods for producing wire-shaped electrochemical supercapacitors by twisting two fiber electrodes together resulted in systems with inferior performance that prevented them from being brought to the market. Lithium ion batteries can attain significantly higher energy density, but have not previously been produced in wire form. In addition to other barriers, the safety problems associated with lithium ion batteries really come into play. The source of the safety problem is dendritic lithium, which can form during over-charging, “growing” out of the anode and causing a short circuit. This can cause the battery to ignite. This seems especially critical for wire-shaped batteries that can be stretched, twisted, and bent during use.
Special Structure and Materials
A team led by Huisheng Peng, Fudan University, Shanghai, China, has now succeeded in producing wire-shaped lithium ion batteries that have a high energy density and are also safe. Their success results from the special structure as well as the materials used. The anode and cathode are two fibers made of parallel multiwalled carbon nanotubes that contain either lithium titanium oxide (LTO) or lithium manganese oxide (LMO) particles, respectively. When the battery is charging, lithium ions are transferred from the LMO lattice to the electrolyte and then into the LTO lattice of the anode. The reverse process occurs as the battery is being discharged. Because the Li insertion takes place at ~1.5 V (vs. Li/Li+) for the applied LTO composite electrode, the chance of short circuit caused by dendritic lithium would be small and therefore the batteries are safe.
The parallel arrangements of continuous carbon nanotubes hold the nanoparticles; they are also efficient pathways for charge transport and serve as current collectors. The two electrode yarns are arranged in parallel, separated by a layer of insulator, and enclosed in a heat-shrinkable tube. To make the wires elastic, they can be wrapped around an elastic fiber such as polydimethylsiloxane and coated with a thin-layer gel electrolyte. Neither repeated stretching to twice its original length nor thousands of deformation cycles reduces the battery capacity.
The wire-shaped batteries can be spun into long fibers and woven into a fabric that can be incorporated into textiles.
- Elastic and Wearable Wire-Shaped Lithium-Ion Battery with High Electrochemical Performance,
Jing Ren, Ye Zhang, Wenyu Bai, Xuli Chen, Zhitao Zhang, Xin Fang, Wei Weng, Yonggang Wang, Huisheng Peng,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201402388