Ruthenium-catalyzed azide-alkyne cycloaddition (RuAAC) is an effective and easy means for the regioselective preparation of 1,5-disubstituted 1,2,3-triazoles and 3,4-disubstituted isoxazoles from terminal alkynes and organic azides or nitrile oxides. RuAAC has had an immediate impact on organic chemistry and related fields, in that the substitution pattern of the resulting products are a perfect complement to triazoles obtained by way of the well-known copper-catalyzed (CuAAC) click reaction, that is, a 1,4-disubstituted 1,2,3-triazole. Although RuAAC has not yet become as robust as CuAAC, its ability to engage internal alkynes in cycloadditions is a distinct advantage, thus providing direct access to fully substituted triazoles and isoxazoles, often with high regioselectivity.
Valery V. Fokin and co-workers, The Scripps Research Institute, La Jolla, CA, USA, have developed a catalytic method for engaging 1-haloalkynes in ruthenium-catalyzed ([RuCl(cod)(Cp)], 1,5-cod = cyclooctadiene, Cp = cyclopentadienyl) azide-alkyne (RuAAc) and nitrile oxide-alkyne cycloaddition reactions to give a convenient and regioselective access to 4-haloisoxazoles and 5-halotriazoles.
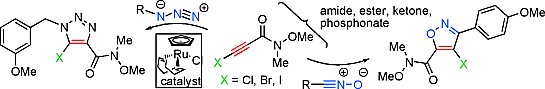
These are important and synthetically useful aromatic heterocycles. Reactive alkynes include 1-chloro, bromo and iodo derivatives and must contain an electron-withdrawing group, such as an amide, ester, ketone, or phosphonate. The product azoles can be further derivatized by catalytic cross-coupling reactions, thus exploiting the potential of the halogen substituent.
- Ruthenium-Catalyzed Cycloaddition of 1-Haloalkynes with Nitrile Oxides and Organic Azides: Synthesis of 4-Halo-Isoxazoles and 5-Halo-Triazoles,
James S. Oakdale, Rakesh K. Sit, Valery V. Fokin,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201402559