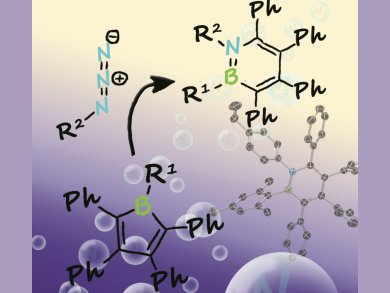
Boron–nitrogen heterocycles, such as azaborinines, are interesting for applications in biomedical systems, organic semiconductors, and hydrogen-storage materials owing to their close resemblance to benzene. However, despite increasing interest, only very few routes have been developed for the synthesis of these compounds.
Holger Braunschweig, Julius-Maximilians-Universität Würzburg, Germany, and co-workers have developed an efficient method for the synthesis of highly substituted monocyclic 1,2-azaborinines by ring expansion of boroles with azides. The reaction allows the use of different substituents at both heteroatoms and even sterically demanding groups, such as mesityl, are tolerated. Interestingly, the electronic properties of the synthesized azaborinines were found to be related to the substituents at the boron and nitrogen centers and also to the substituents in the backbone of the heterocycle.
With the starting boroles becoming increasingly available and a wide variety of azides already established, this synthetic method could lead to many new functional organoboron materials.
- Antiaromaticity to Aromaticity: From Boroles to 1,2-Azaborinines by Ring Expansion with Azides,
Holger Braunschweig, Christian Hörl, Lisa Mailänder, Krzysztof Radacki, Johannes Wahler,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201403101