The fabrication of photocontrolled molecular machines is one of the significant topics in supramolecular chemistry. To date, a great number of artificial molecular machines with promising photophysical properties, such as molecular shuttles and molecular switches, have shown potential applications in chemical and biological fields.
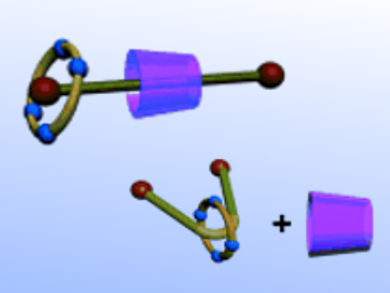
Previously, Yu Liu and co-workers, Nankai University, Tianjin, China, have constructed a reversible lanthanide luminescence switch dual-modulated by an optical stimulus and the selective complexation of dibenzo-24-crown-8 with a dialkylammonium guest. Taking advantage of the complementary molecular-binding selectivity of α-cyclodextrin and tetrasulfonated 1,5-dinaphtho-32-crown-8, the same group reports the synthesis of a photoresponsive [3]pseudorotaxane in water. The interconversion from a heterowheel [3]pseudorotaxane to a [2]pseudorotaxane can be effected by the photoinduced isomerization of pyridinium-modified azobenzene as the molecular axle.
The researchers think that the study on these light-controlled multicomponent assemblies will guide them in the construction of more sophisticated nanostructures in the future.
- Light-Controlled [3]Pseudorotaxane Based on Tetrasulfonated 1,5-Dinaphtho-32-Crown-8 and α-Cyclodextrin,
Jun Wang, Ying-Ming Zhang, Xu-Jie Zhang, Xiao-Jun Zhao, Yu Liu,
Asian J. Org. Chem. 2015.
DOI: 10.1002/ajoc.201402238