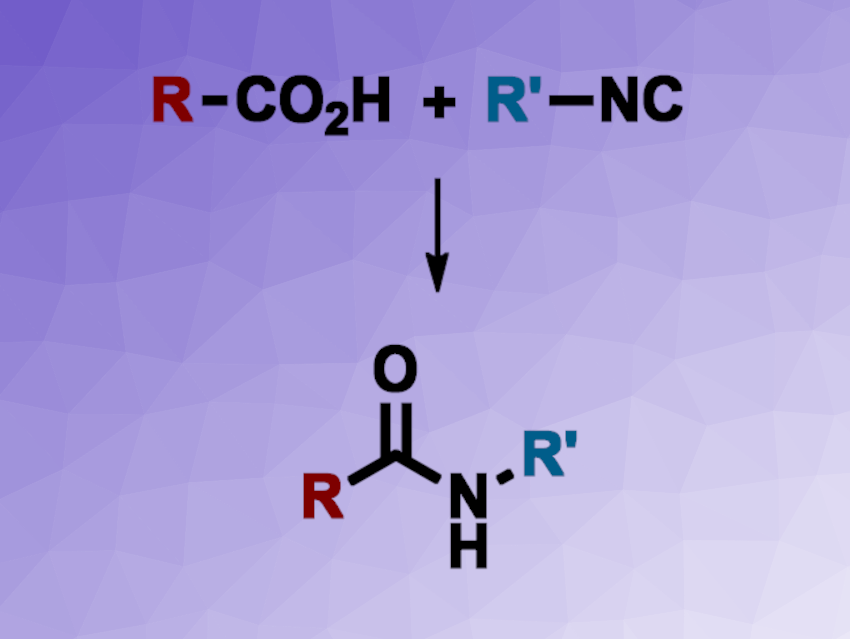
Amides are not only important in proteins and peptides in nature, but also, for example, in synthetic polymers or pharmaceutically active compounds. They are often synthesized from carboxylic acids and amines using activating agents, which is generally not optimal from a sustainability/green chemistry standpoint. New methods for the synthesis of amides with high atom efficiency, are, thus interesting.
Jian-Quan Liu, Xiang-Shan Wang, Jiangsu Normal University, China, Markus D. Kärkäs, KTH Royal Institute of Technology, Stockholm, Sweden, and colleagues have developed a method for the silver-catalyzed decarboxylative cross-coupling of carboxylic acids and isocyanides to obtain amides via a free-radical mechanism. The team reacted aryl or alkyl isocyanides with a wide variety of carboxylic acids using Ag2CO3 as a catalyst in a 20:1 mixture of acetone and water under air at 60 °C.
Under these conditions, the desired amides were obtained in high yields. The researchers successfully used the approach for the late-stage functionalization of bioactive compounds. They propose a reaction mechanism that involves the silver-catalyzed decarboxylation of the carboxylic acid to give a radical. The radical then undergoes a coupling to the isocyanide (either as a free isocyanide or as a silver complex), giving a radical imine adduct. This is followed by an oxidation using O2 from the air to give a peroxide, removal of one oxygen atom, and a protonation to obtain the amide. Overall, the method provides a general approach to amides with good functional group tolerance.
- General Approach to Amides through Decarboxylative Radical Cross-Coupling of Carboxylic Acids and Isocyanides,
Qing Yan, Qing-Jia Yuan, Andrey Shatskiy, Gregory R. Alvey, Elena V. Stepanova, Jian-Quan Liu, Markus D. Kärkäs, Xiang-Shan Wang,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00872