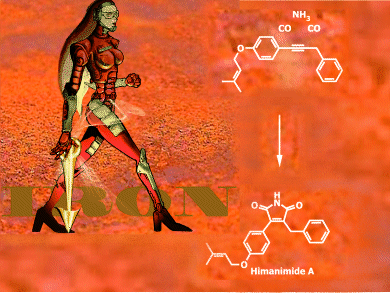
Through the power of iron catalysis, the syntheses of himanimide A and B have been conveniently achieved in only four and five steps, respectively, to afford the desired natural products from commercially available starting materials.
As described in the Communication by Matthias Beller et al., Universität Rostock, Germany, an iron-catalyzed [2+1+1+1]-annulation strategy was used for the construction of five-membered maleimides, which are building blocks for naturally occurring himanimides A and B. While himanimide B was prepared for the first time, the synthesis of himanimide A has been significantly improved with respect to overall yield. This synthetic strategy can be applied to other related bio-active compounds, such as camphorataimides, antrocinnamomins, polycitrins, and arcyriarubins.
- Iron-Catalyzed Carbonylation as a Key Step in the Short and Efficient Syntheses of Himanimide A and B
S. Prateeptongkum, K. M. Driller, R. Jackstell, M. Beller
Chem. Asian J. 2010, 5.
DOI: 10.1002/asia.201000384