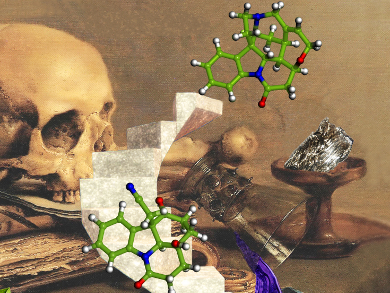
The formation of two or more bonds in one reaction vessel, known as cascade or domino processes, represents an efficient strategy for the construction of complex natural products. The development of increasingly sophisticated, yet simple and scalable cascade reactions has received considerable attention from the organic chemistry community in the last decades. Synthetic planning of old and new target molecules is now dominated by the concept of step-, atom-, and redox economy. The synthesis of strychnine, a historically important member of the indole alkaloid family, has become a testing ground for new synthetic strategies.
Hans-Ulrich Reissig and Christine Beemelmanns, Freie Universität Berlin, Germany, describe a highly diastereoselective samarium diiodide-mediated cascade cyclization of substituted indolyl ketones that leads to one of the most efficient synthetic approaches to the legendary alkaloid strychnine. The complexity-generating transformation with SmI2 allows two six-membered rings and three stereogenic centers, including a quaternary center, to be generated in the key step.
All selectivity problems encountered in the preparation of the crucial strychnine precursor isostrychnine were elegantly bypassed in this efficient synthesis. The group currently explores the reaction scope of SmI2-induced cascade cyclizations and of in situ functionalizations.
- Evolution of a Short Route to Strychnine by Using the Samarium Diiodide-Induced Cascade Cyclization as a Key Step,
Christine Beemelmanns, Hans-Ulrich Reissig,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201500094
Also of Interest
- Agatha Christie: The Chemistry of a (Nearly) Perfect Murder,
Klaus Roth,
ChemViews Mag. 2015.
DOI: 10.1002/chemv.201500022 - Strychnine: From Isolation to Total Synthesis – Part 1,
Klaus Roth,
ChemViews Mag. 2015.
DOI: 10.1002/chemv.201500031